Spina Bifida
Spina bifida is a defect resulting from failure of closure of the posterior neuropore, occurring anywhere along the spinal axis, but most commonly involving the lumbosacral region (63% of cases). Exposure of neural elements to amniotic fluid and meconium may lead to severe functional impairment. Spina bifida can be categorized as (1) meningocele (sac with CSF only), usually occurring at the upper and lower ends of the neural axis (i.e. occipital, cervical, and low sacral positions), or (2) myelomeningocele (protrusion of a sac containing CSF and neural elements), typically located at the lower thoracolumbar, lumbar, and lumbosacral areas. In addition, spina bifida can also be divided into open (80%), defined as either uncovered or covered with a thin translucent membrane, and closed (20%), i.e. covered with skin or a thick, opaque membrane.
Fig 1, Fig 2, Fig 3
Incidence: Approximately 1 in 1000 births, but it varies greatly from 0.3/1000 in Japan to 4-8/1000 births in the UK; it also depends on specific risks such as familial history.
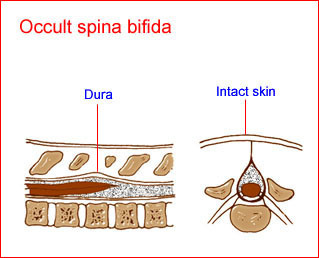
Fig 1: Schematic drawing of spina bifida occulta: a defect of the spine with intact skin and no alteration of the spinal cord
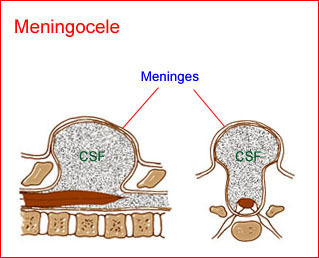
Fig 2: Schematic drawing of spina bifida : meningocele; protrusion of meninges and cerebrospinal fluid (CSF) through the defect
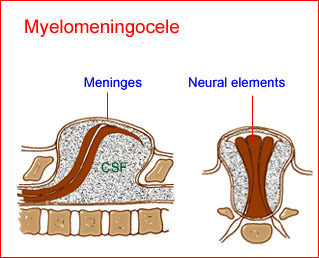
Fig 3: Schematic drawing of spina bifida : myelomeningocele; pro-trusion of neural elements with meninges and cerebrospinal fluid (CSF) through the defect
Sonographic findings:
Direct signs Fig 4, Fig 5, Fig 6, Fig 7, Fig 8, Fig 9
- Visualization of the spinal dysraphic defect; widely separated ossification centers of the posterior processes, best visualized on the transverse scan (the coronal scan is also helpful). In the coronal plane, distortion of the vertebral architecture with spina bifida results in disappearance of the central line and widening of the two lines.
- Demonstration of myelomeningocele sac, which is present in the majority of cases, usually covered by a thin translucent membrane and containing both CSF and neural elements, best visualized on the sagittal scan (both coronal and transverse scans are also helpful). In the sagittal scans, the posterior line and overlying soft tissues are absent at the level of the lesion and these scans are also useful for evaluating the severity of the lesion as well as spinal curvatures that may be exaggerated with large defects.
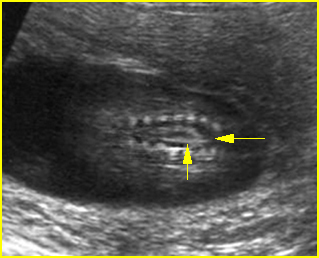
Fig 4: Spina bifida Coronal scan of the lumbosacral spine: separation of the ossification centers of the spine (arrow)
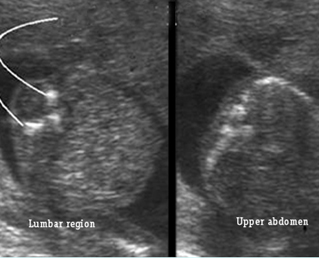
Fig 5: Spina bifida Cross-sectional scan of the lumbar and thoracic spine: separation of posterior ossification centers at the lumbar region with small protrusion of the overlying tissue
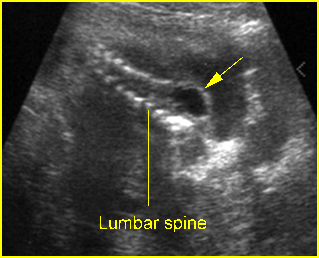
Fig 6: Lumbar meningocele Oblique sagittal scan of the spine: small cystic mass (arrow) protruding from the lumbar region
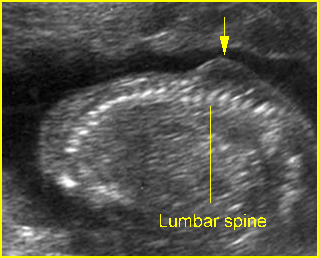
Fig 7: Lumbar meningocele Oblique sagittal scan of the spine: small protrusion (arrow) of the overlying skin at the lumbar region
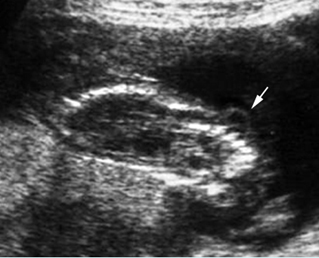
Fig 8: Lumbar meningocele Oblique sagittal scan of the spine: small cystic mass (arrow) protruding from the lumbar region
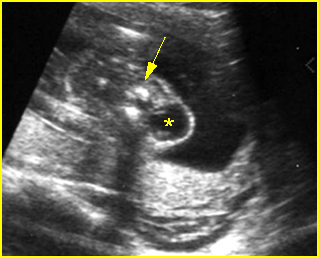
Fig 9: Cervical meningocele Cross-sectional scan of the cervical spine: small cystic mass (*) arising from cervical spine (arrow)
Video clips of spina bifida
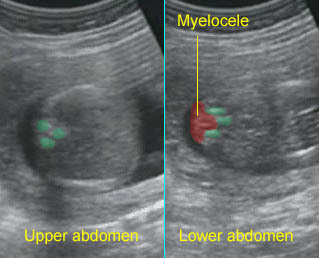
Spina bifida Cross-sectional scan of the normal spine at the level of upper abdomen, but wide separation of the posterior ossification centers at the lumbar spine with the protrusion of the spinal cord
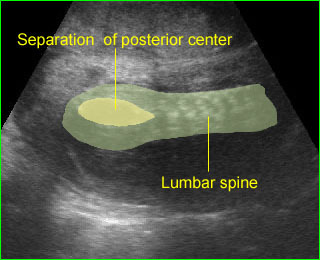
Spina bifida : Coronal scan: mild separation of the posterior centers of the lumbosacral spine
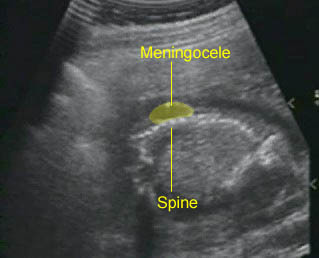
Spina bifida : Sagittal scan: small meningocele at the lumbar spine
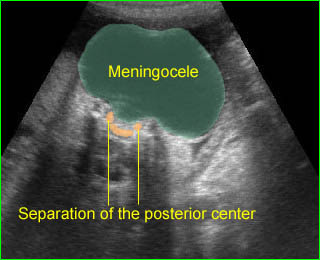
Large meningocele : Cross-sectional scan of the normal spine at the level of upper abdomen, but wide separation of the posterior ossification centers at the lumbar spine with the protrusion of the meningeal sac
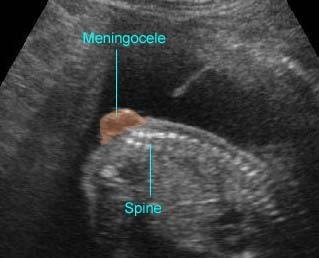
Spina bifida : Sagittal scan of the fetal spine showing sacral meningocele
Indirect signs
Fig 10, Fig 11
- Lemon sign: a concave deformity or scalloping of the frontal bones at the level of the coronal suture which is best elicited on axial scans at the level of lateral ventricles. The lemon sign is a very sensitive sign for predicting spina bifida before 24 weeks with a sensitivity of 98%, but it has a low sensitivity (13%) after 24 weeks. It is not specific and is found in 1-2% of normal fetuses.
- Cerebellar sign: obliteration of the cisterna magna caused by the caudal displacement of the cranial contents resulting in either an absent cerebellum or a flattened, centrally curved, banana-like cerebellum (banana sign). Cerebellar signs are highly sensitive (95%) irrespective of gestation; however, the sign at <24 weeks of gestation is predominantly the banana sign (72%), whereas at gestations >24 weeks it is cerebellar “absence” (81%). The effacement of the cisterna magna has a high negative predictive value and is useful during routine screening of the fetal neural axis.
- Ventriculomegaly: about 80% of fetuses with spina bifida demonstrate ventriculomegaly by 24 weeks.Most fetuses with open spina bifida develop ventriculomegaly, and the majority do so by 21 weeks of gestation.
- Talipes develops after 20 weeks in most cases.
- The sensitivity of the cranial signs is approximately 99% with a false-positive rate of 0-3%.
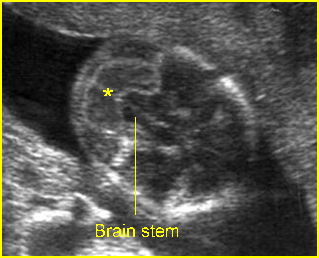
Fig 10: Banana sign Transcerebellar view: banana shape (*) of cerebellum, instead of dumbbell shape, occurring in lumbar meningocele
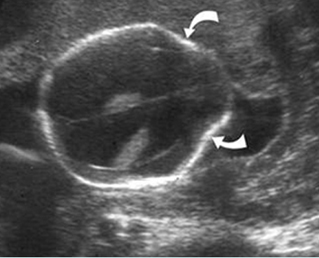
Fig 11: Lemon sign Transverse scan at the level of lateral ventricles: indent frontal bones in association with lumbar meningocele
Video clips of spina bifida
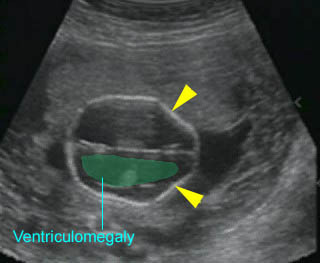
Lemon sign : Indentation of the frontal bones (arrowhead) with ventriculomegaly
– The accuracy of the diagnosis depends on the experience of the operator, and the quality of the equipment varies from 70% to nearly 100%. However, the sensitivity among routine non-targeted examinations is probably about 75-80%. With an experienced operator, sonography alone has a sensitivity of 97% and a specificity of 100%.
– Normal ultrasound is an appropriate basis for a reduction of at least 95% in the maternal serum alpha-fetoprotein-based risk for neural tube defects.
The main differential diagnosis includes
- sacrococcygeal teratoma
- conjoined twins, especially with large tumors
- hemangioma
- neuroectodermal cyst.
– Usually diagnosed in the second trimester, but possibly detected at 9-10 weeks with transvaginal ultrasound.
Pitfalls:
- Oblique views through the lumbar spine can make the gluteus muscle look like a myelomeningocele. Three-dimensional ultrasound is helpful in this case.
Associations: Spina bifida is an isolated abnormality in most cases. However, 9-17% of cases are associated with abnormal chromosomes, especially trisomy 18 and 13. Associated anomalies other than neuromuscular deformities due to the spinal defect include hydrocephalus (most common) associated with Arnold-Chiari malformation, ACC, DWM and other extracranial anomalies.
Management: Termination of pregnancy can probably be offered when diagnosed before viability. In continuing pregnancies, delivery can occur at term. Preterm delivery may be considered in cases of rapid development of ventriculomegaly. Prelabor cesarean section may be considered to reduce motor dysfunction. Although several reports have indicated that in utero repairs of myelomeningocele decrease hindbrain herniation and shunt-dependent hydrocephalus, the operation seems to be associated with an increased rate of premature delivery, no improvement in the degree of paralysis and bladder function, and possibly pulmonary hypoplasia. The procedures should be considered as investigational.
Prognosis: Depends on the size, location and the presence of ventriculomegaly, the extent of the lesion, and the presence or absence of neural tissue. Although modern surgical and medical management has resulted in better long-term function, the mortality rate and urinary tract and orthopedic disability are sill high.
Recurrence risk: Isolated neural tube defects, a multifactorial disorder, carry a recurrence risk of about 2-4% after one affected pregnancy, and 10-15% after two affected pregnancies. Folic acid periconceptional supplementation can effectively reduce the recurrent risk. 50-70% of these defects can be prevented if a woman consumes sufficient folic acid daily before conception and throughout the first trimester of her pregnancy.

