Gastroschisis
Gastroschisis
Gastroschisis is a paraumbilical defect involving all the layers of the abdominal wall. It is usually right-sided with the small bowel herniated through the defect. The stomach or other organs may occasionally be involved in herniation.
Fig 1
Incidence: 1 in 2000-5000 live births with a high prevalence among the maternal age of 11-19 years. It has been associated with various medications including recreational drugs, NSAIDs (aspirin, salicylate, ibuprofen), and decongestants (pseudoephedrine and phenylpropanolamine) . There has been a sustained increase in the incidence over the past decade, particularly in teenage women.
Sonographic findings:
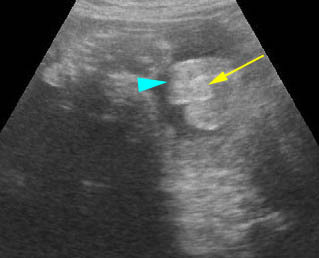
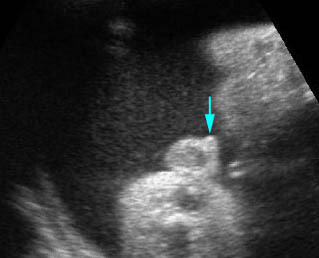
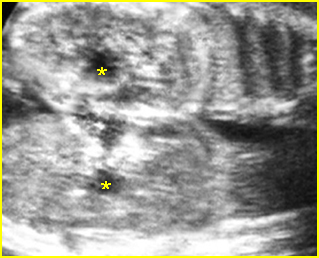
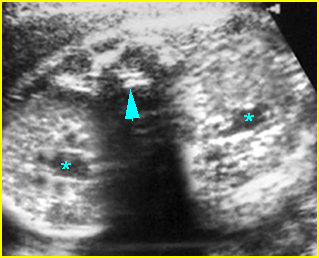
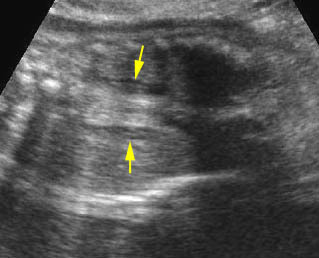
- Abdominal organs herniate through an anterior abdominal wall defect; typically, there are multiple loops of bowel outside the abdomen. Fig2, Fig3, Fig4
- No covering membrane.
- Umbilical cord inserting on the abdominal wall (normal insertion site). Fig5, Fig6
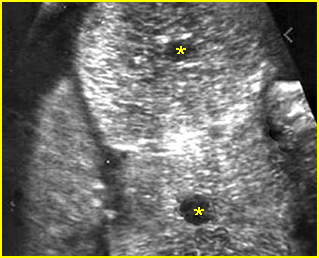
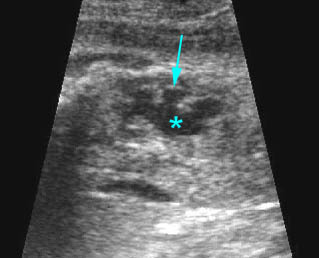
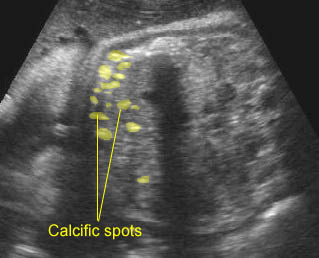
- Varying degrees of bowel dilatation and wall thickening or increasing intraluminal meconium. Fig7, Fig8, Fig9
- The liver is very rarely involved. Fig10
- The amniotic fluid volume is usually normal, however, oligohydramnios may be observed in 36% of cases. Polyhydramnios may be seen in the case of bowel obstruction.
- An increase in serum alpha-fetoprotein levels, higher than those associated with omphalocele.
- Doppler velocity of the superior mesenteric artery found to be predictive of outcome in fetuses with gastroschisis.
- Usually first diagnosable in the early second trimester; the diagnosis prior to 13 weeks may be confused with physiologic herniation.
- Pitfalls: The cases with oligohydramnios, particularly in late pregnancy, can easily be missed. Additionally, extra-abdominal bowel can be overlooked and thought to be a coiled umbilical cord.
- Differential diagnoses include other types of abdominal wall defects (see omphalocele), especially ruptured omphalocele, though rare, limb-body wall complex, and tangled cord adjacent to the fetal abdomen, in which color flow will show vascular flow.
- Most standard ultrasound parameters are not significantly associated with an adverse neonatal outcome, except for polyhydramnios, which was strongly predictive of severe bowel complications in the neonatal period.
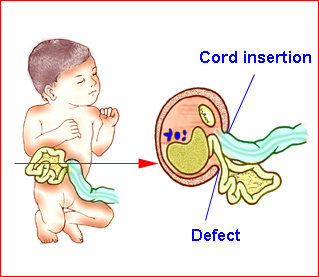
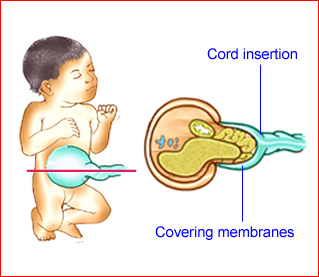
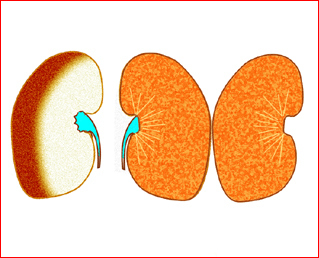
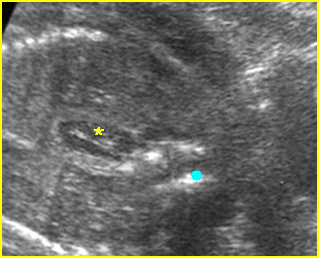
Fig 1: Schematic drawing of gastroschisis; note normal location of the cord insertion
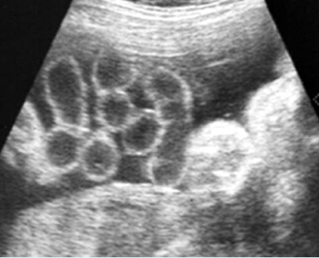
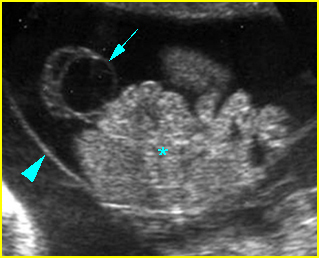
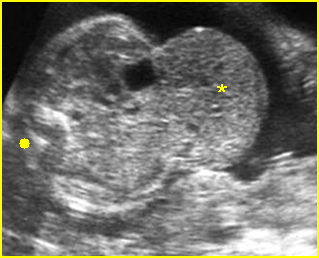
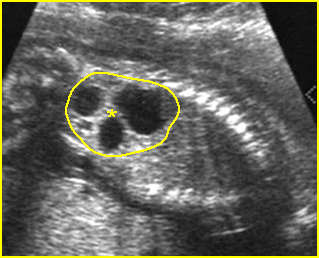
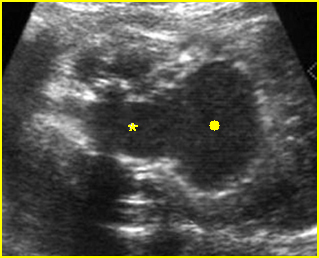
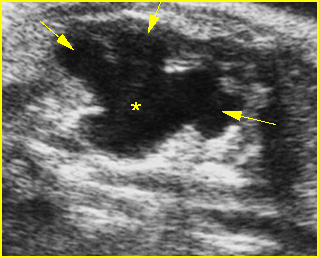
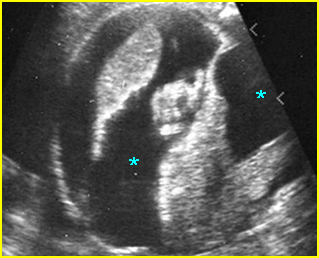
Fig 2: Gastroschisis Scan of the free-floating bowel loops in the amniotic fluid
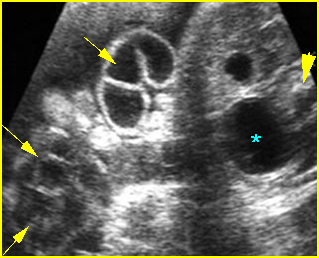
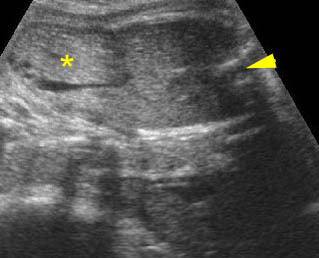
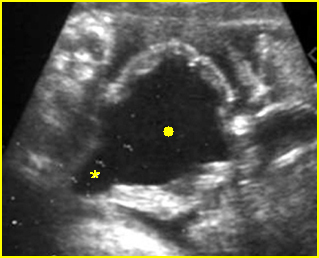
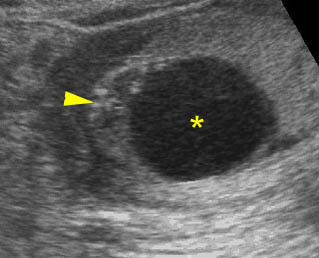
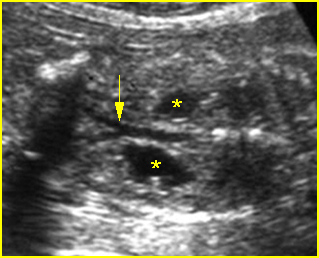
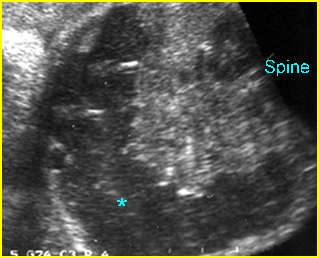
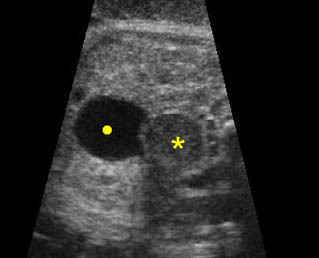
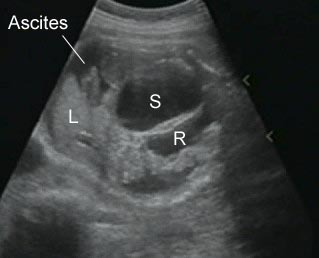
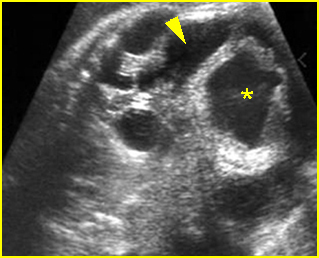
Fig 3: Gastroschisis Cross-sectional scan of the abdomen: Free floating dilated bowel loops (arrow) in the amniotic fluid (arrowhead = spine, * = intra-abdominal stomach)
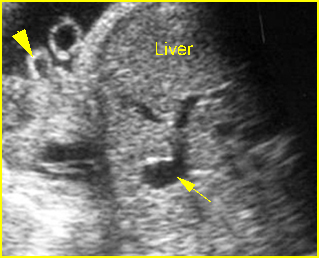
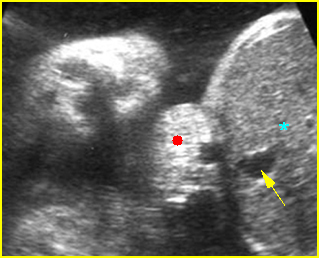
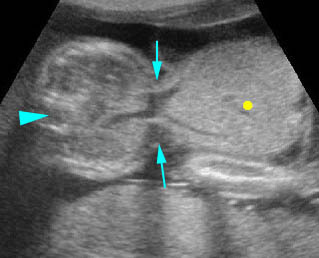
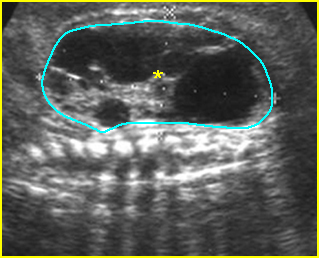
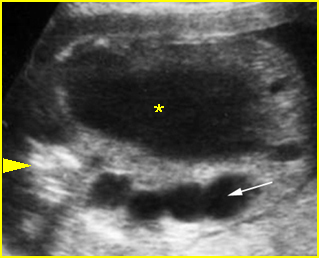
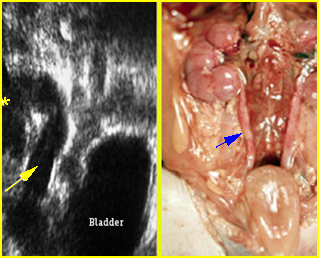
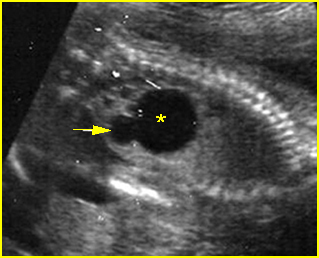
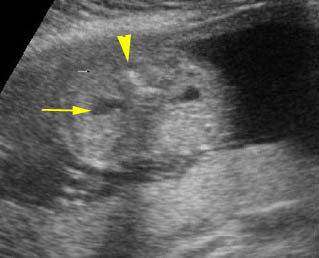
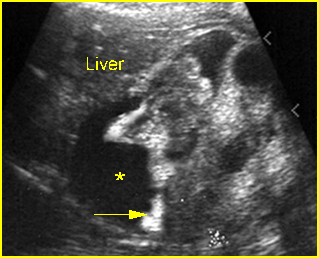
Fig 4: Gastroschisis Cross-sectional scan of the abdomen: bowel (arrowhead) protruding through the abdominal wall defect on the right-side of the umbilicus (arrow = umbilical vein)
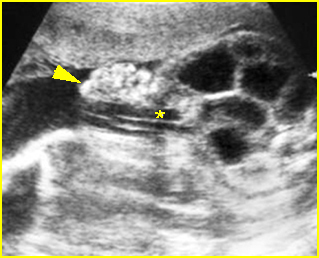
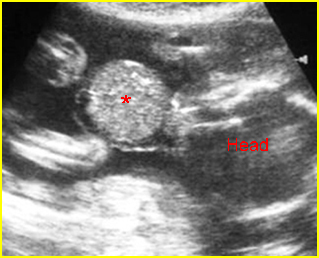
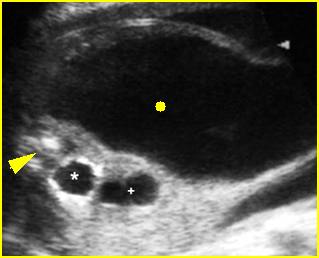
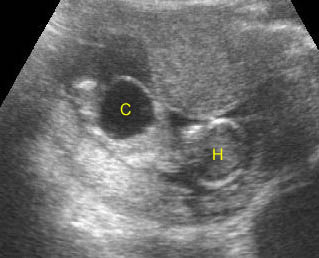
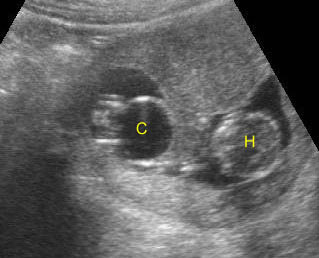
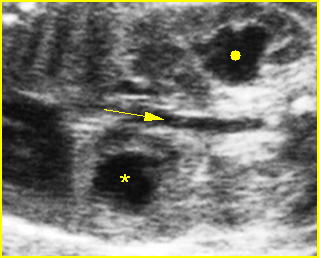
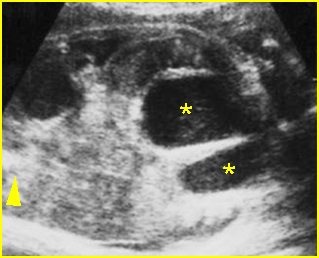
Fig 5: Gastroschisis Cross-sectional scan of the abdomen: free floating bowel in the amniotic fluid on the right side of the umbilical vessels (*) protruding through the abdominal wall defect
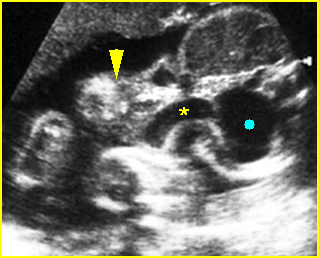
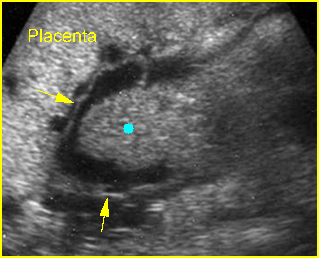
Fig 6: Gastroschisis Cross-sectional scan of the abdomen: Free floating bowel loops (arrow) in the amniotic fluid, protruding through the abdominal wall defect on the right-side of the umbilical vein (*) (solid circle = intra-abdominal stomach)
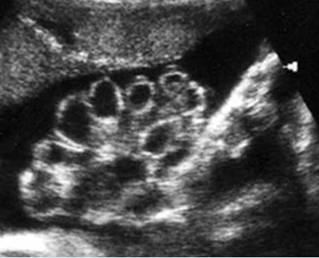
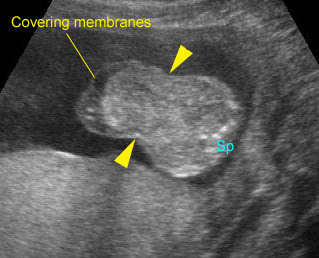
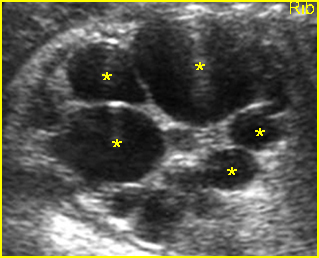
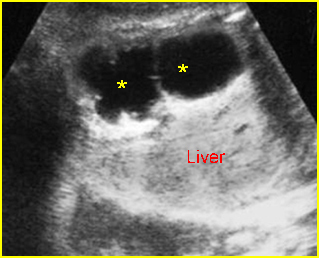
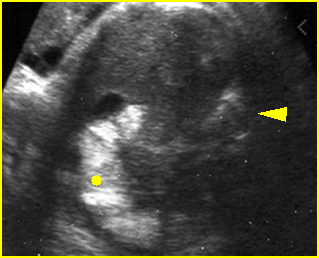
Fig 7: Gastroschisis Free floating dilated bowel loops in the amniotic fluid
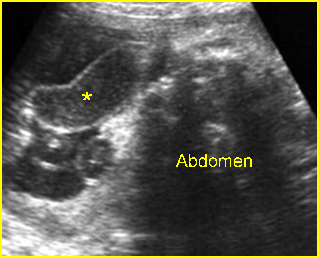
Fig 8: Gastroschisis Free floating dilated bowel loops (*) in the amniotic fluid
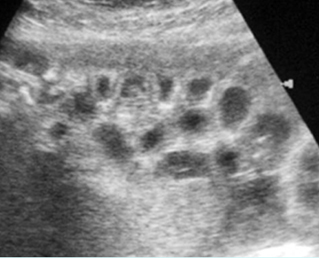
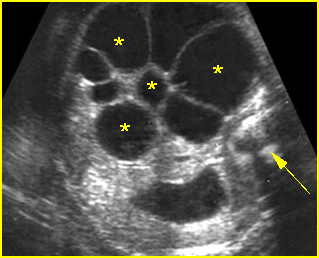
Fig 9: Gastroschisis Free floating bowel loops (*), thickened wall caramelized by the turbid amniotic fluid
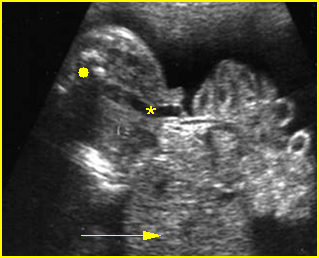
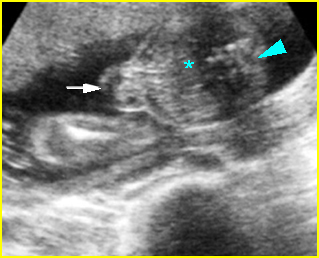
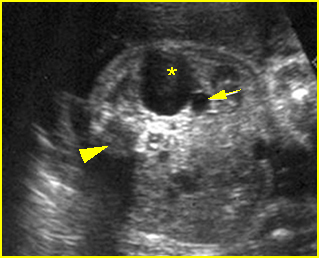
Fig 10: Gastroschisis Cross-sectional scan of the abdomen: liver mass (arrow) and bowel (arrowhead) without covering membrane protruding through large defect, note normal cord insertion (*) (solid circle = spine)
Video clips of gastroschisis
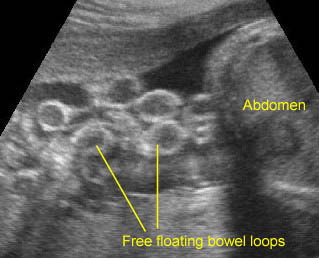
Gastroschisis: Free floating bowel loops with mild dilatation
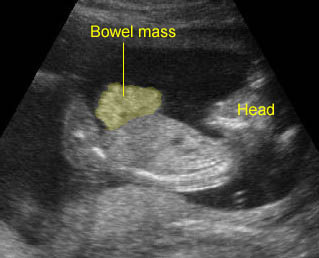
Gastroschisis: Free floating bowel mass anterior to abdominal wall
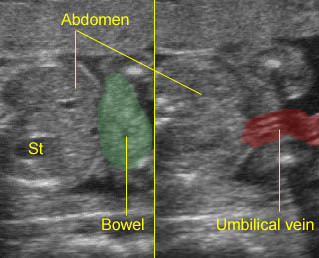
Gastroschisis: Free floating bowel mass anterior to the abdominal wall and normal cord insertion
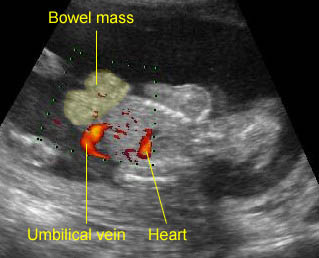
Gastroschisis: The defect is located at the right to the normal cord insertion
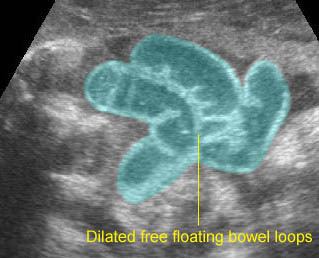
Gastroschisis: Dilated free floating bowel loops representing some degree of obstruction
Associations: Related to other anomalies in 7-10% of cases, mostly GI anomalies, not associated with chromosomal abnormalities.
Management: Serial sonographic monitoring is necessary to evaluate the degree of dilation and wall thickness. Early delivery may be beneficial in cases of marked dilatation or thickened bowel wall, however, term delivery gives the best outcomes in most cases. Delivery should be performed in a tertiary center with appropriate facilities for surgical management of the newborn. The fetuses may carry a higher rate of fetal distress and intrauterine death and fetal surveillance is recommended, however, labor and ruptured membranes do not appear to be associated with increased neonatal morbidity or mortality rates in neonates with gastroschisis. Cesarean section probably does not improve the outcomes.
Prognosis: Good but complications are very common, especially those related to the gastrointestinal tract problems and prematurity, increased morbidity in cases of bowel obstruction, bowel wall thickening, or increased amniotic concentration of digestive compounds.
Recurrence risk: Sporadic except for rare familial cases.