Normal Examination
Technique of examination
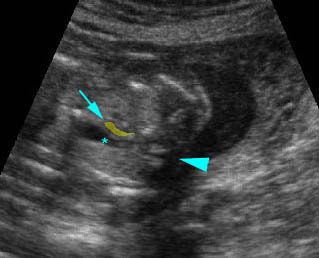
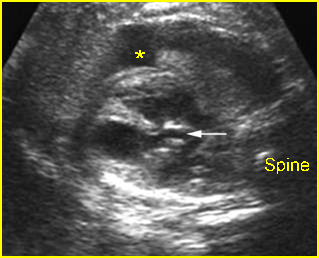
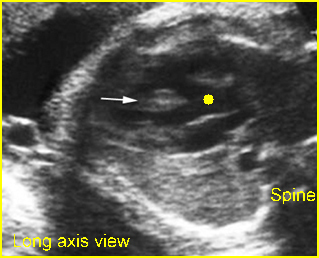
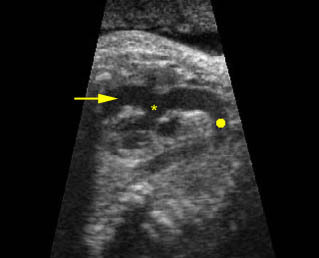
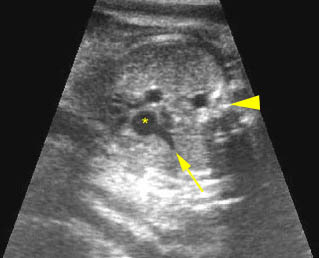
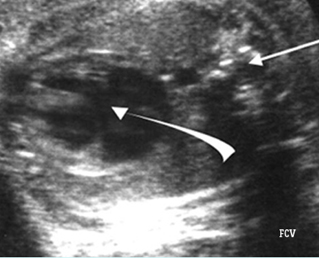
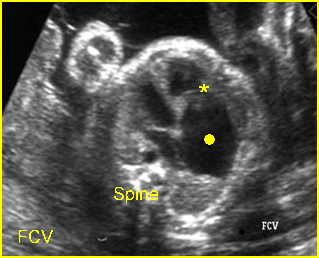
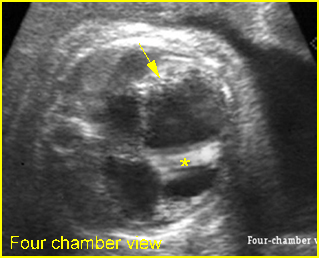
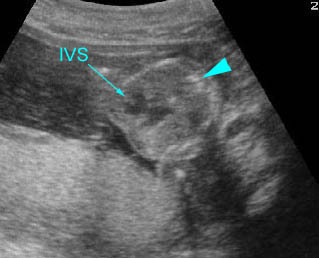
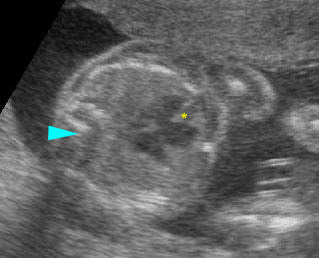
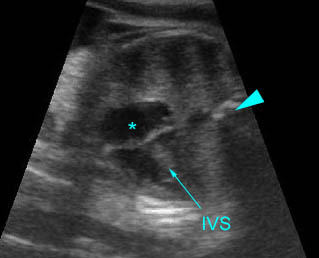
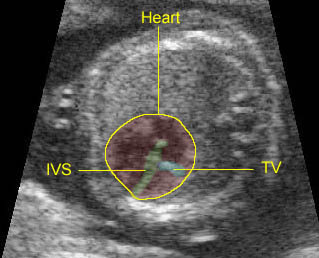
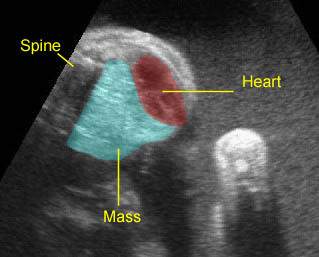
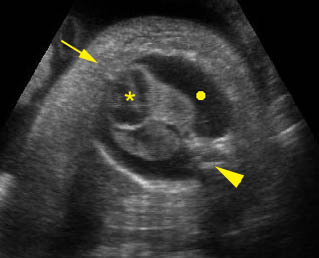
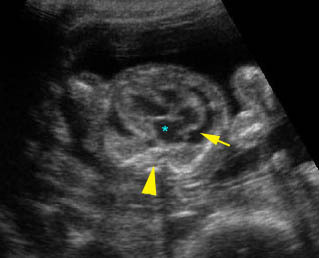
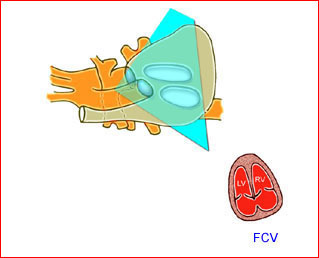
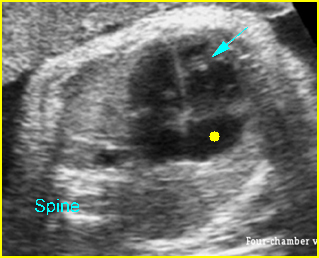
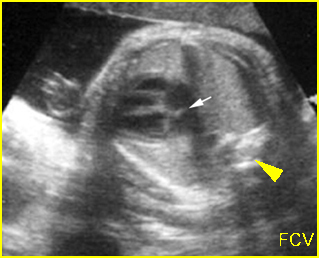
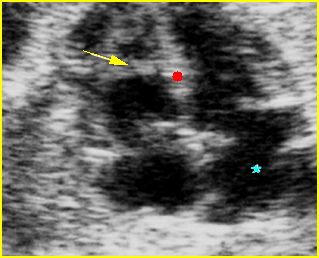
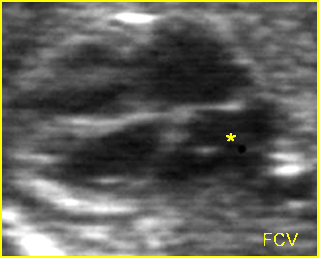
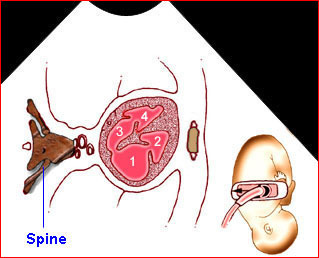
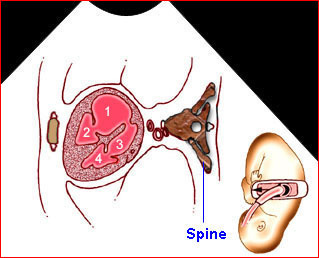
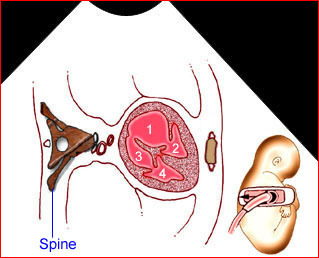
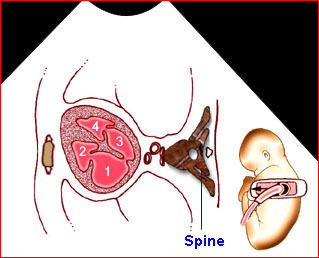
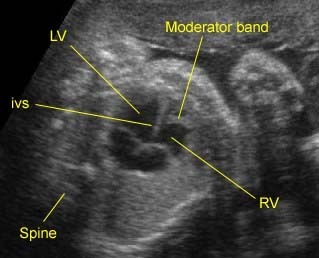
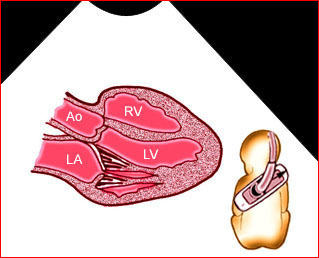
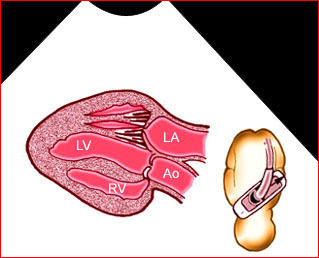
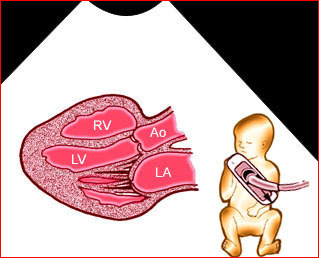
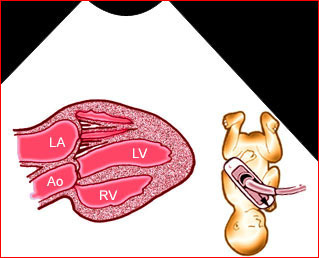
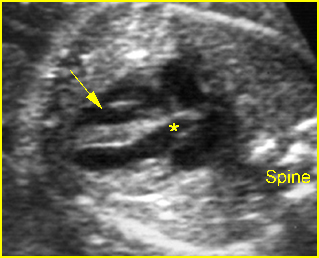
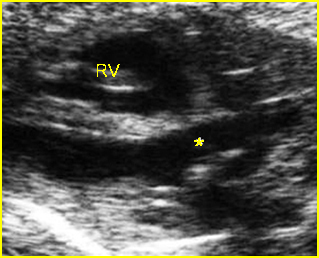
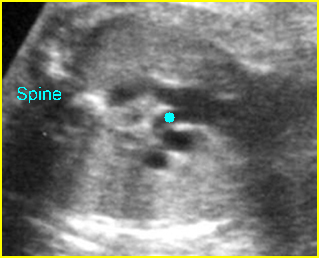
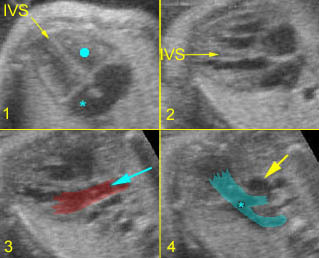
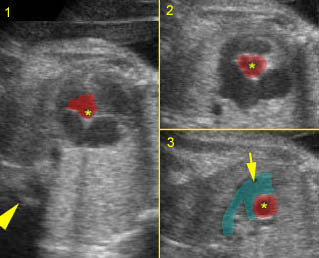
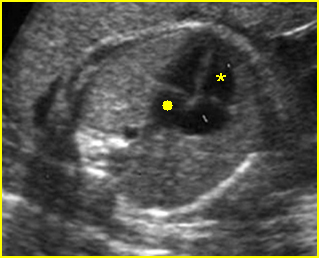
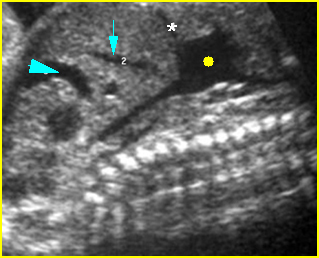
– Transverse thoracic plane: Fig 1, Fig 2, Fig 3 The ultrasound transducer should be oriented to visualize the upper thorax first, from clavicles, and then move down to the abdomen. A sweep in transverse planes provides important information, including the ribs, thoracic spine, lung echogenicity, cardiac location, size, and axis. The four-chamber view can also be satisfactorily assessed. This view should be examined in all fetuses in the second and third trimesters as part of a routine scan. The thorax is rather round on this plane. The plane at the level of the four-chamber view can also perform thoracic circumference measurements.
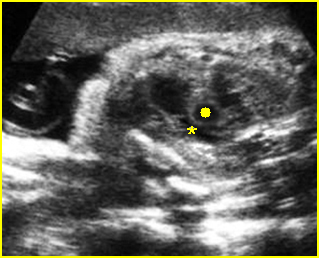
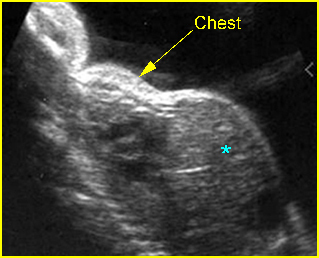
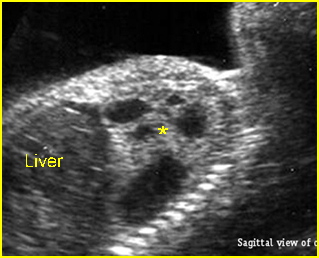
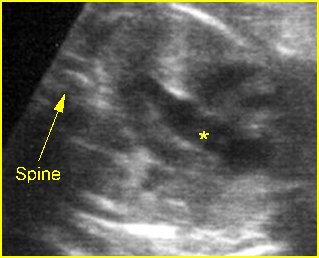
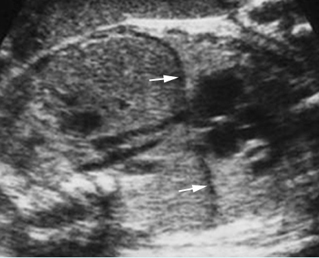
– Sagittal thoracic plane: Fig 4 The transducer should be adjusted to obtain the anteroposterior images from one side to the other. The thorax appears dome-shaped on this plane. The diaphragm and thoracic size compared with the abdomen can be assessed.
– Coronal thoracic plane: Fig 5, Fig 6 The transducer is oriented to obtain the coronal images from the spine to the anterior wall. The thorax appears bell-shaped on this plane. The diaphragm and thoracic size compared with the abdomen can be assessed.
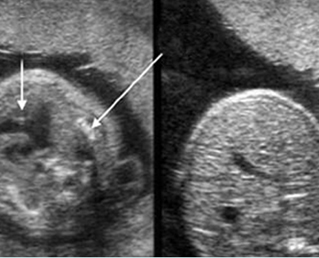
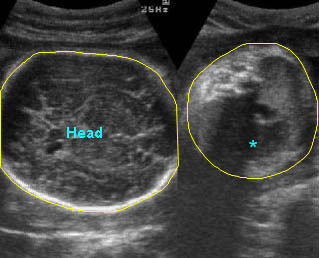
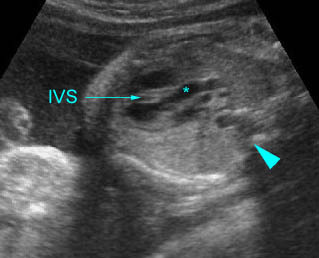
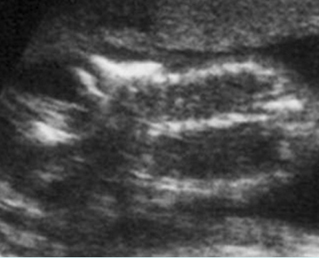
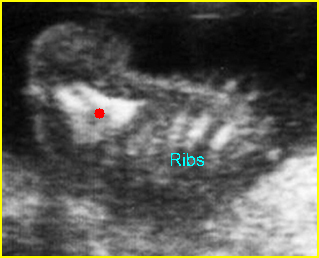
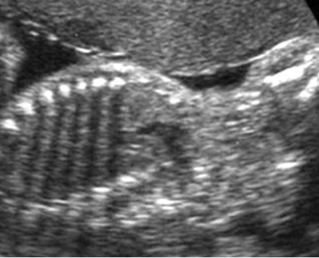
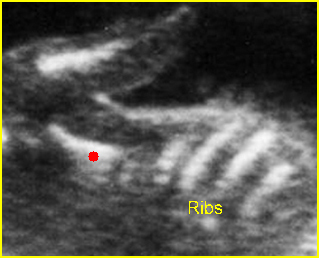
– Bones assessment: Fig 7, Fig 8, Fig 9, Fig 10 The fetal thoracic cage is bell-shaped and bordered by the clavicles (which can also be measured) at the apices and the smooth hypoechoic diaphragm inferiorly. The ribs, smoothly marginated and regularly spaced, form the lateral boundaries and extend anteriorly and inferiorly more than halfway around the thorax from their dorsal attachments. The subjective impression of the thoracic size is sufficient in most cases. However, direct measurement of the thoracic circumference may be of value in the diagnosis of lung hypoplasia.
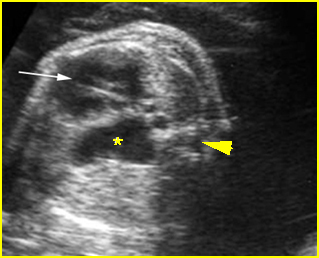
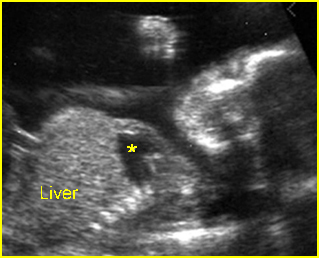
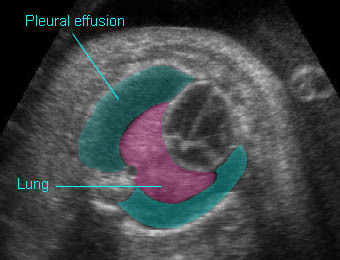
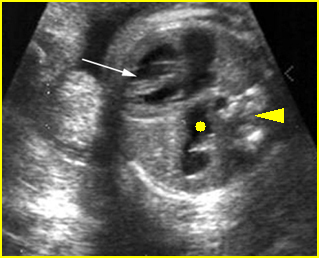
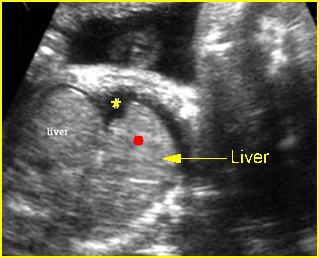
– Lungs: Fig 11 The lungs have homogeneous mid-range echoes, however, the echogenicity may be greater than, less than, or equal to the echogenicity of the liver. The lungs may become progressively more echogenic than the liver as gestation progresses. No abdominal contents should be seen with the lungs.
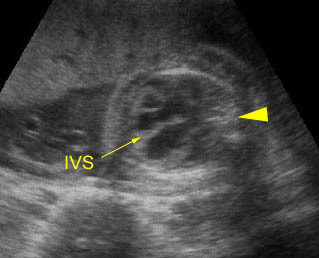
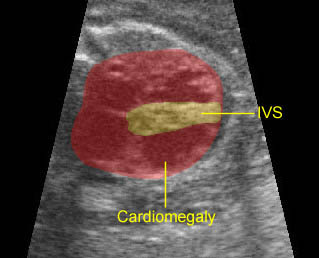
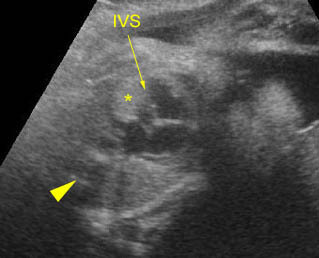
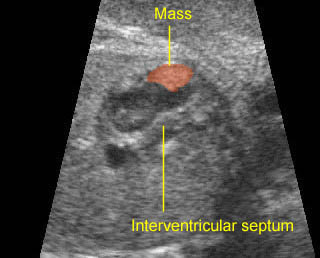
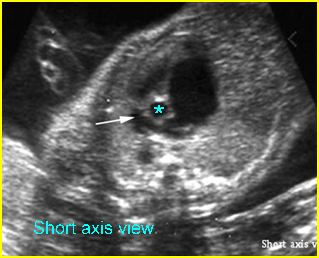
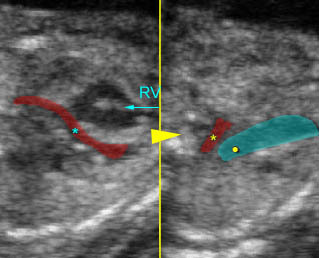
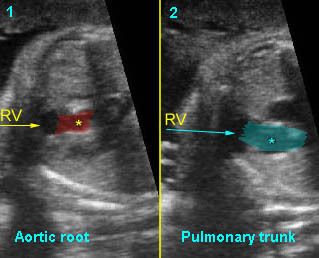
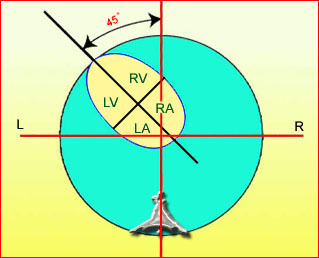
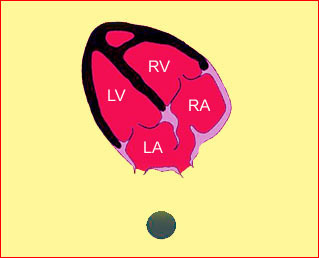
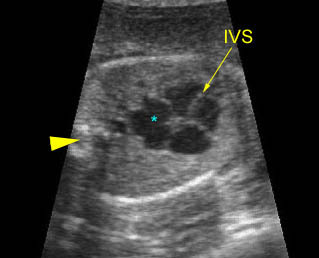
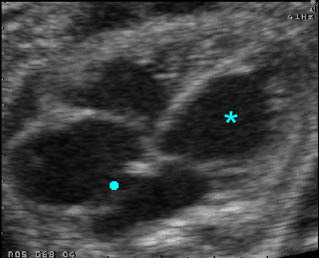
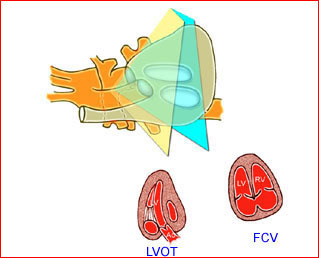
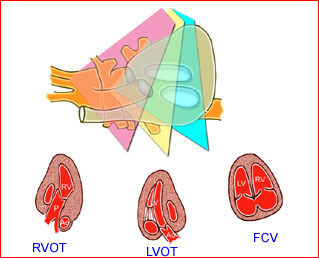
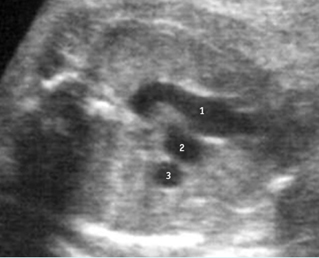
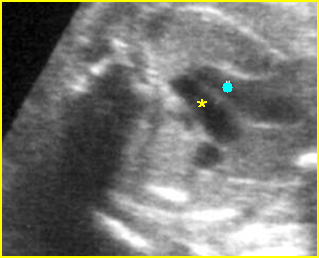
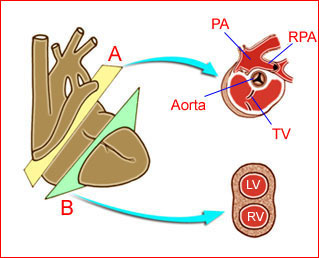
– Heart: Fig 2, Fig 3 On the transverse plane, the visualization of the normal four-chamber view of the heart with appropriate cardiac position, axis, and size helps to exclude many thoracic abnormalities. The four-chamber view examination can detect 60-70% of cardiac defects and an additional view of the ventricular outflow tracts and great vessels further increases that sensitivity. The heart occupies about one-third of the thoracic volume, and most of the cardiac volume is located in the left anterior quadrant of the chest. The cardiac axis, the angle that the interventricular septum makes with the line drawn from the spine to the anterior chest wall, ranges between 22 and 75 degrees, with a mean of 45 degrees. Echogenic foci caused by a specular reflection from the papillary muscles and chordae tendinae are commonly seen. The bright foci, termed echogenic intracardiac foci, can be associated with chromosome abnormalities.
– Thymus: The thymus, a homogeneous, relatively hypoechoic structure in the anterior fetal mediastinum, could be visualized with some effort sonographically in 74% of 251 normal fetuses.
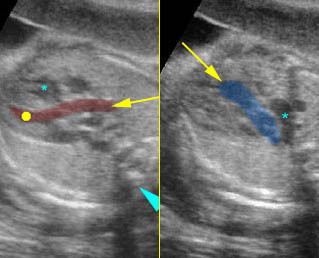
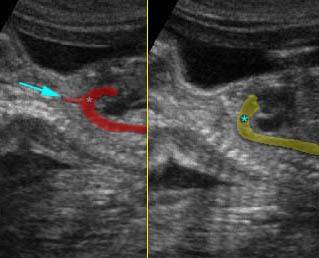
– Larynx and Trachea: Fig11 The larynx is virtually always visible when the hypopharynx is fluid-filled. The fluid-filled trachea is a relatively easy structure to visualize. The fetus intermittently and frequently breathes amniotic fluid into the trachea. The trachea usually can be traced to its distal end, passing posterior to the aortic arch, but the carina and bronchi are quite difficult to perceive.