Pleural Effusion / Chylothorax
Pleural Effusion/Chylothorax
Pleural effusion is an accumulation of fluid in the pleural space. Effusion may be either clear (hydrothorax) or chylous. It is mostly related to hydrops fetalis secondary to various causes. Primary chylothorax is the most common cause of an isolated pleural effusion, leading to respiratory distress in newborns.
Incidence: Primary hydrothorax occurs in approximately 1 per 15,000 pregnancies.
Sonographic findings:
Fig 1, Fig 2, Fig 3
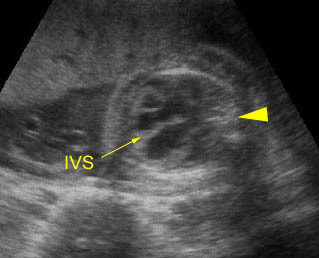
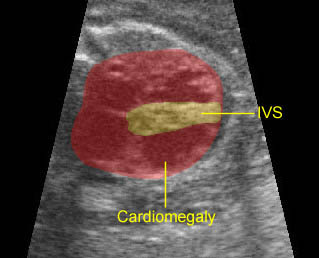
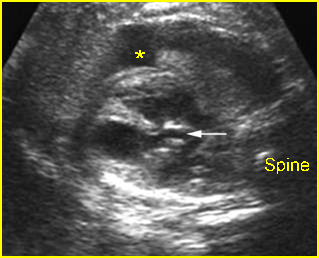
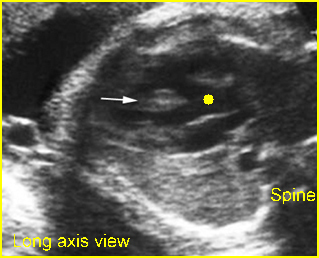
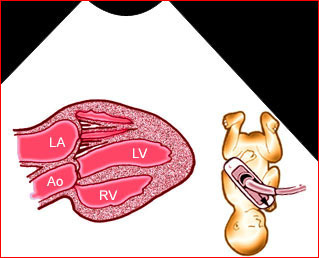
- Sonolucent or anechoic space around the lungs and heart.
- Other findings related to hydrops fetalis such as ascites, cystic hygroma or polyhydramnios.
- Chylothorax is diagnosed when pleural effusion is isolated, the size of the effusion is relatively larger than other effusions, and the effusion occurs before the development of the hydrops, if present. However, the diagnosis relies on the postnatal characteristic milky appearance resulting from the chylomicrons in the lymph fluid.
- Primary fetal hydrothorax may resolve or progress to hydrops, necessitating close follow-up with ultrasound.
- Echocardiographic assessment, including the measurement of the effusion ratio, may be a useful tool in guiding fetal therapy.
- Usually diagnosed in the second and third trimesters, though some case are detected in the first trimester.
Associations: Various causes of hydrops fetalis particularly secondary to Hb Bart’s disease, primary chylothorax may be related to trisomy 21 or Turner syndrome.
Management: A conservative approach with a follow-up scan in 2-3 weeks is appropriate in most cases of isolated hydrothorax. Thoracocentesis just prior to delivery may be helpful for large effusions. In utero thoracoamniotic shunt can reverse hydrops in some cases. Intrapleural injection of OK-432 was reported to be helpful in a case of chylothorax.
Prognosis: Depends upon the underlying cause; primary chylothorax in the absence of hydrops fetalis has a good prognosis with proper drainage, and eventually resolves in most cases. Poor prognostic factors include the presence of hydrops or lung hypoplasia, bilaterality, and gestational age. Thoracoamniotic shunt may be helpful for large effusion or hydrops. Additionally, embryonic/fetal pleural effusion in early pregnancy was associated with poor pregnancy outcome such as spontaneous abortion and chromosomal abnormality.
Recurrence risk: Low recurrence for isolated cases.
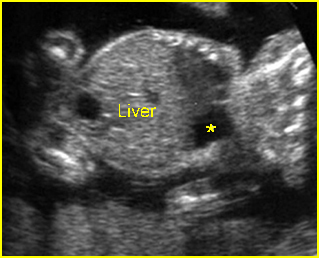
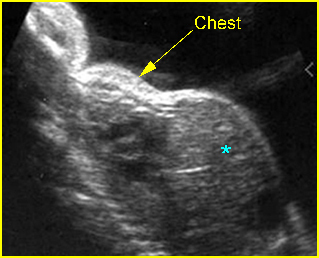
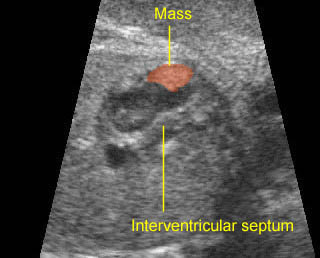
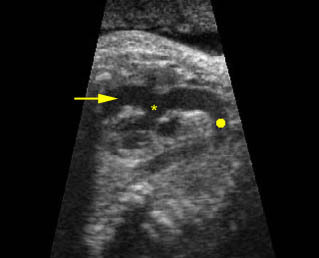
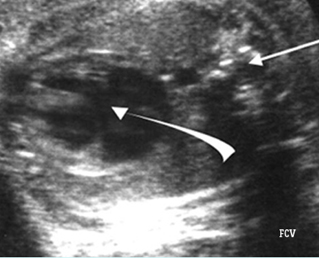
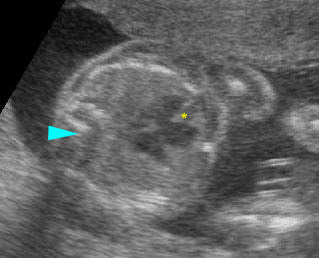
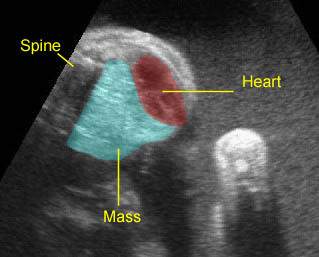
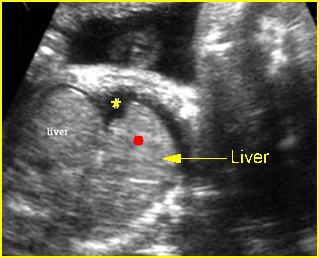
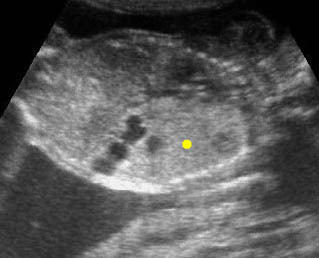
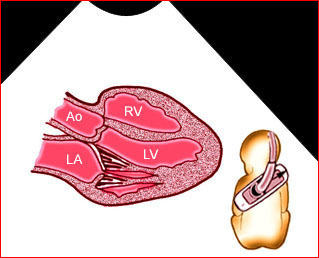
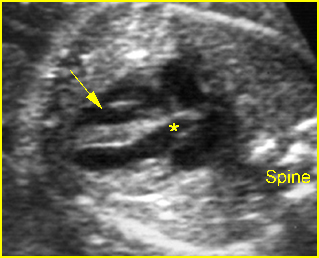
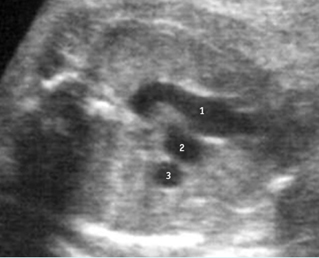
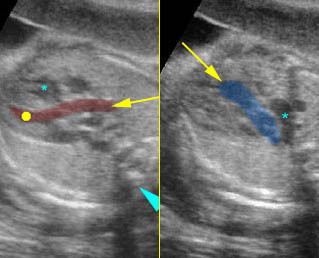
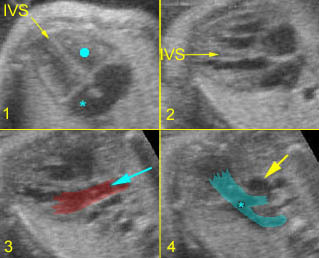
Fig 1: Chylothorax Coronal view of the fetus 21 weeks: pleural effusion (*)
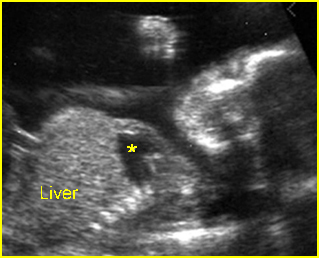
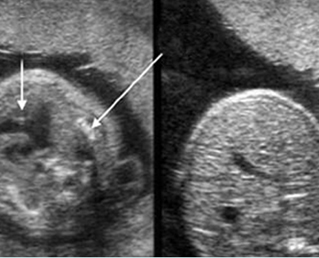
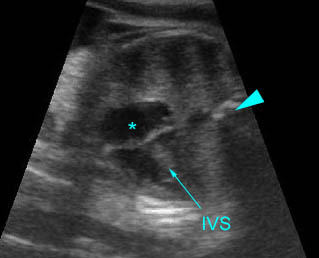
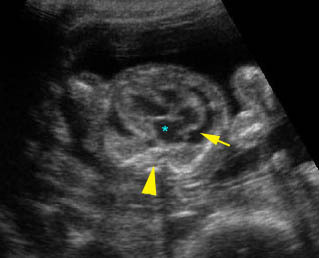
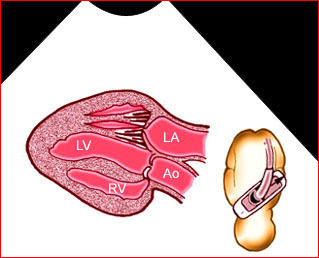
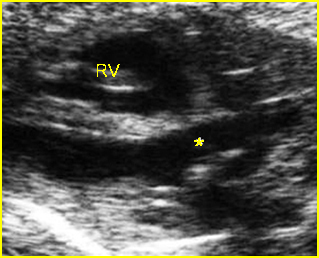
Fig 2: Chylothorax Oblique sagittal view of the fetus 21 weeks: pleural effusion (*)
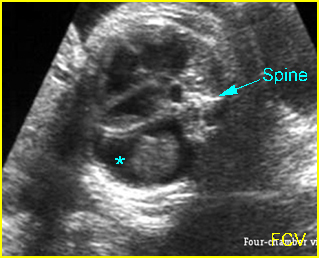
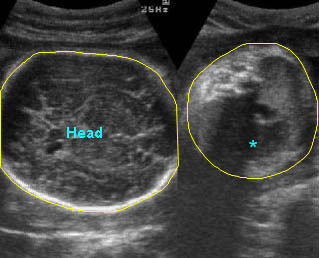
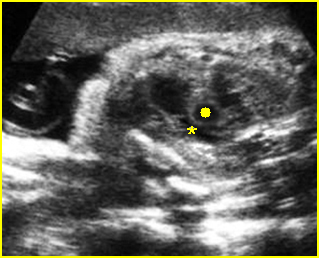
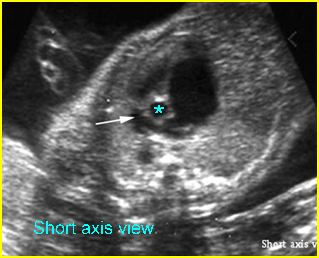
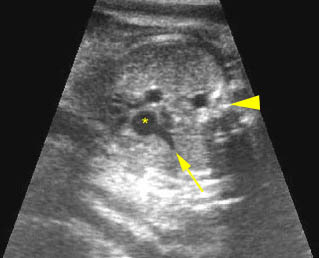
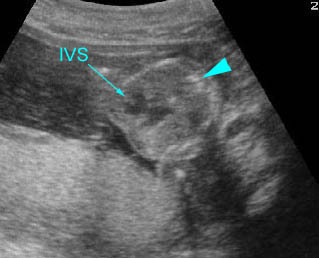
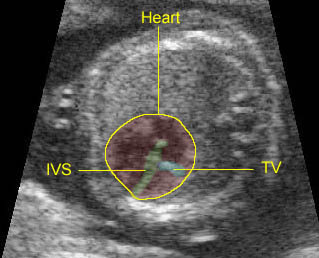
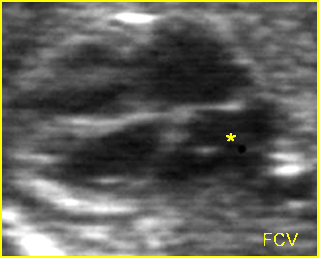
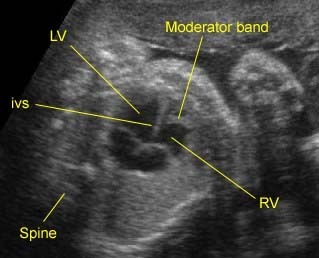
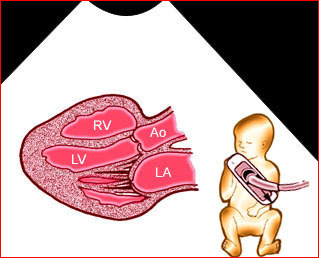
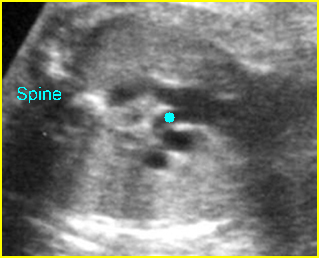
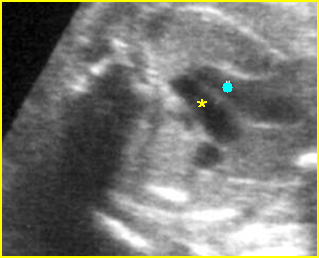
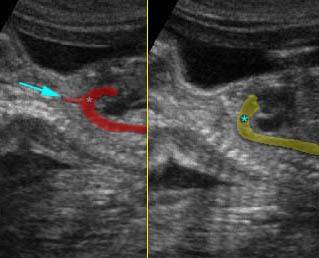
Fig 3: Pleural effusion Cross-sectional scan of thorax: pleural effusion (*) in the left thorax with cardiac displacement to the right
Video clips of pleural effusion / chylothorax
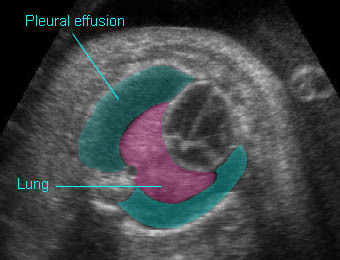
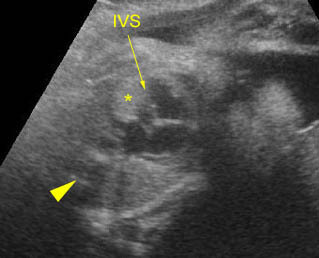
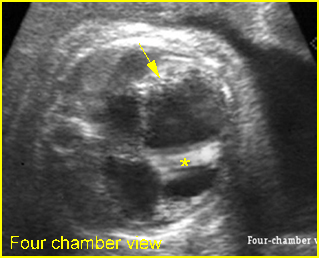
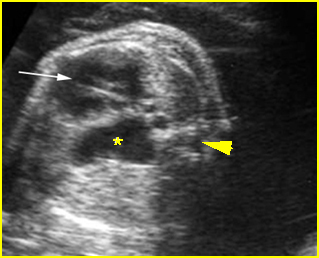
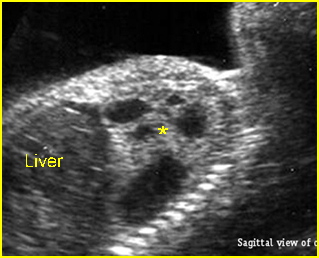
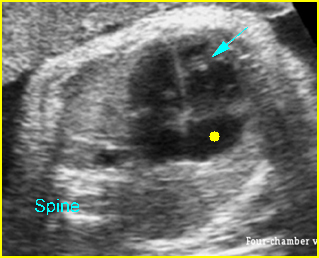
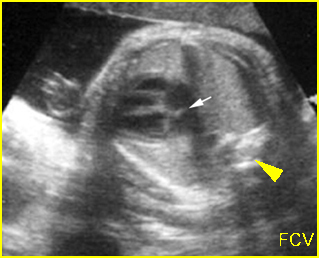
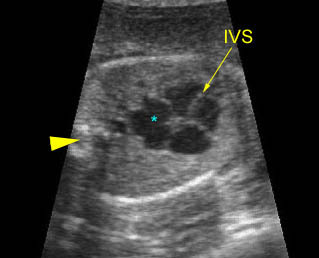
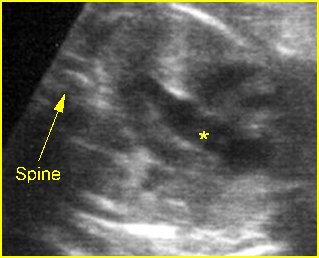
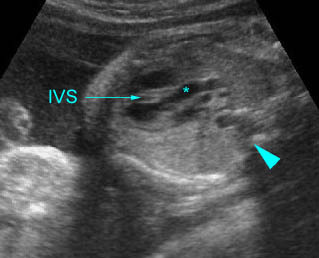
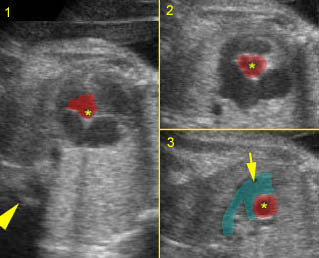
Chylothorax: Four-chamber view: marked pleural effusion (*) and subcutaneous edema (arrowhead), normal cardiac size
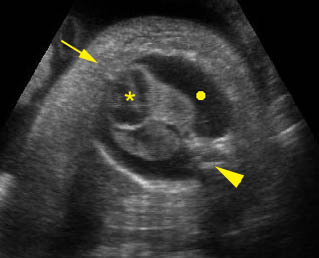
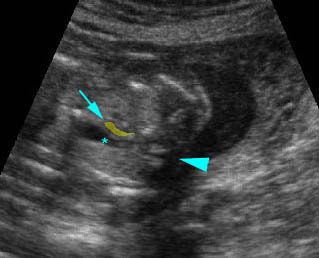
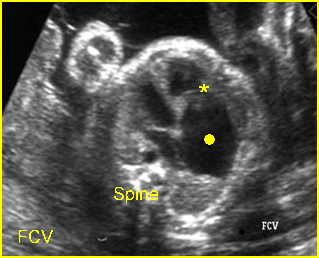
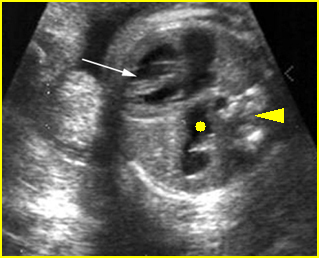
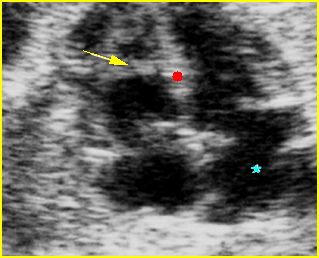
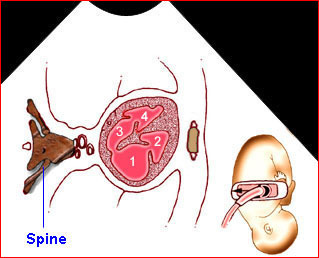
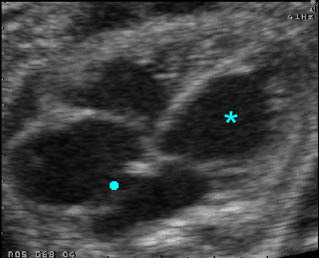
Hydrops fetalis: Four-chamber view: marked pleural effusion (solid circle), normal cardiac size (*), subcutaneous edema (arrow) (arrowhead = spine)