Normal Examination Spine
Normal Examination
Normal Spine
Fig 1, Fig 2, Fig 3, Fig 4, Fig 5, Fig 6
– Mineralization of the spine begins at 8 weeks of gestation. The three ossification centers of individual vertebrae include a single ventral center for the vertebral body (centrum) and two paired dorsal centers that will become the lateral masses and the posterior arch. They are well visualized from 15 to 16 weeks onwards. However, spina bifida can be detected much earlier. The posterior ossification centers begin at the base of the transverse processes. As ossification progresses, the laminae become visible, usually after 19 weeks. The inward angulation of the normal laminae is the opposite of the outward splaying of the laminae seen in spina bifida, an optimal situation for detecting this anomaly. The arch of the upper sacral region is not consistently recognizable until after 25 weeks.
- Technique of examination: The spine can be systematically examined during the second and third trimesters as follows:
- Adjust the transducer on the maternal abdomen to achieve a cross-section image of the fetal abdomen or thorax.
- Identify the location of the fetal spine, noting the acoustic shadow behind the spine, and carefully adjust the transducer to demonstrate all three ossification centers.
- To obtain the sagittal view, from the previous image, make an attempt to bring both posterior centers to the middle of the image, lying horizontally or perpendicular to the ultrasound beam, and then rotate the transducer 90 degrees and meticulously adjust it to obtain the best image of the coronal view.
- To obtain the coronal view, from the cross-section image, make an attempt to bring both posterior centers to the middle of the image, lying vertically or parallel to the ultrasound beam, and then rotate the transducer 90 degrees and meticulously adjust it to obtain the best image of the coronal view.
- Evaluation of the three views of the spine:
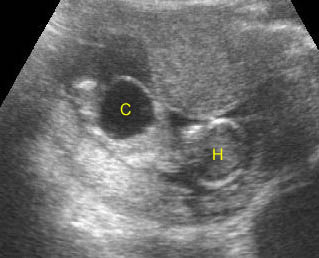
- On the transverse (axial) view, the anterior ossification center (vertebral body) and posterior ossification centers (pedicles, transverse processes, laminae, and spinous processes) may all be identified as echogenic structures. This view may be superior to longitudinal views in demonstrating small spinal defects, since all three ossification centers can be imaged simultaneously.
- On the sagittal view, many vertebrae can be visualized on a single image. The curvature can be best evaluated<?is this sentence complete enough?>. The normal spine appears as two parallel lines formed by the vertebral bodies anteriorly and the ossification centers of the lateral processes converging in the sacrum. The lines correspond to the posterior elements of the vertebrae and the vertebral body. This view can optimally demonstrate interruption of the overlying integument when myelomeningocele is present.
- On the coronal view, many vertebrae can be visualized on a single image. Hence, scoliosis, hemivertebrae, and disorganized vertebrae are optimally visualized on this view. <?can you check the following sentence>The images oriented through the dorsal ossification centers can demonstrate the two parallel rows of echogenicity in these centers and also the extent of a dysraphic defect compared to adjacent vertebrae in the case of spina bifida.
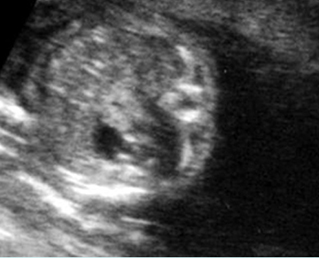
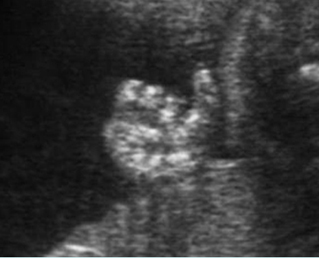
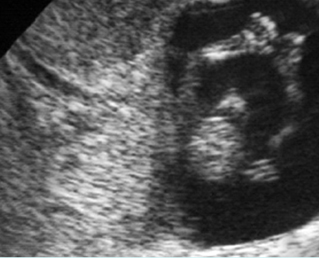
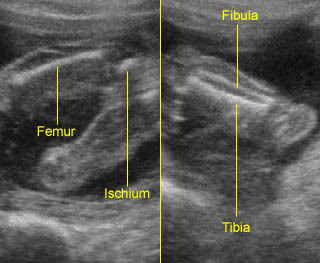
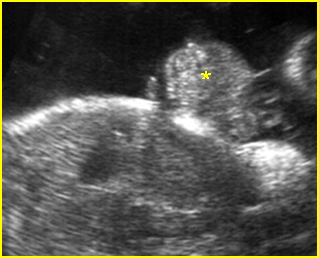
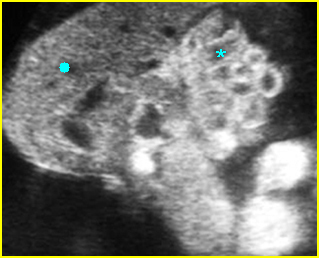
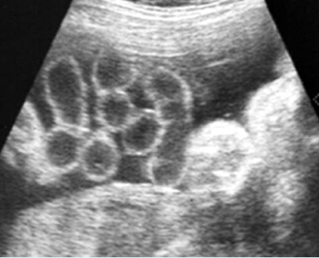
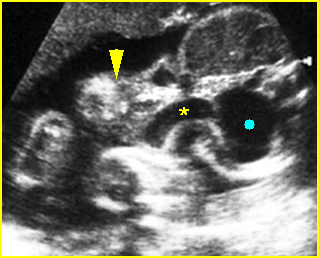
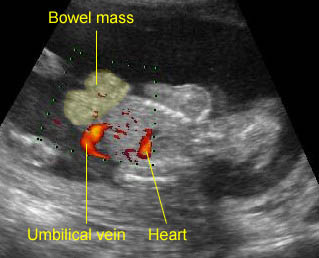
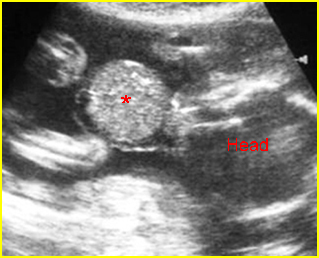
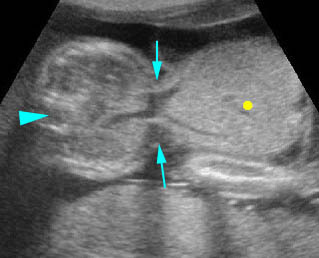
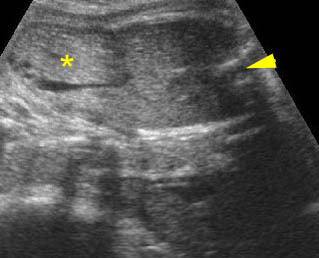
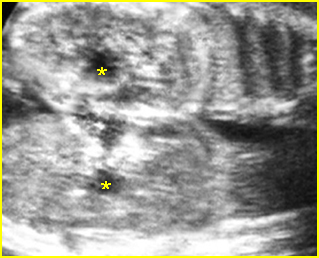
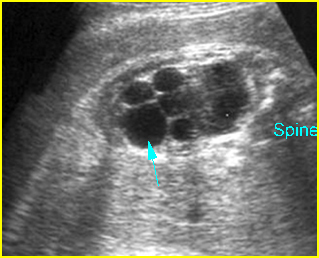
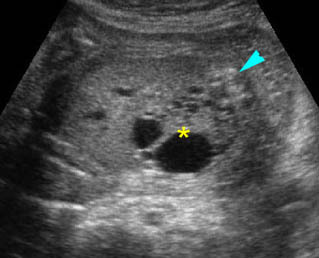
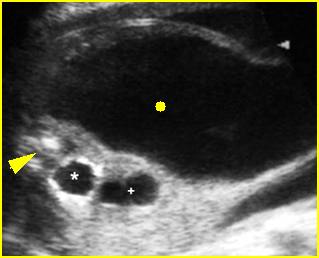
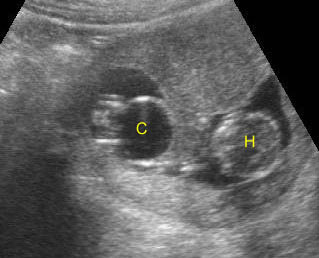
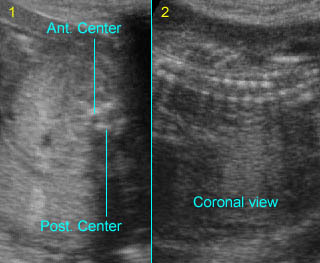
Fig 1: Normal spine Cross-sectional scan of the abdomen: posterior ossification centers of spine align vertically
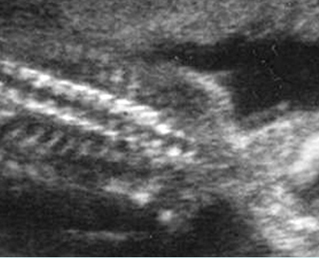
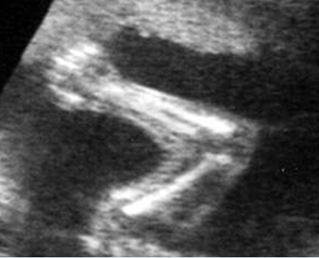
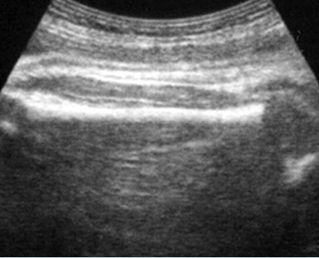
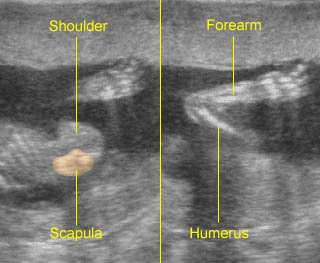
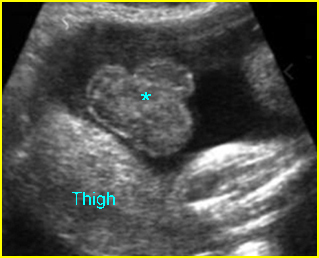
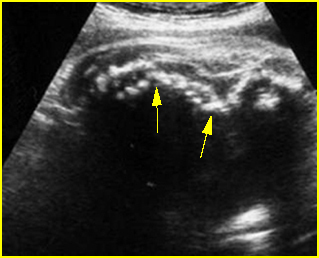
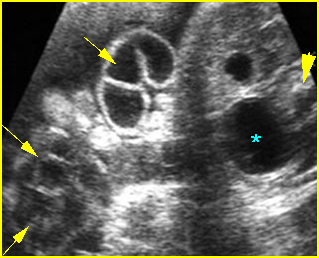
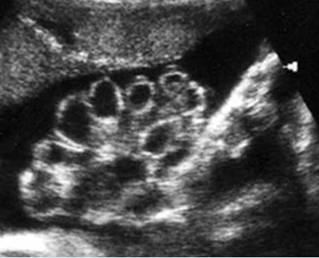
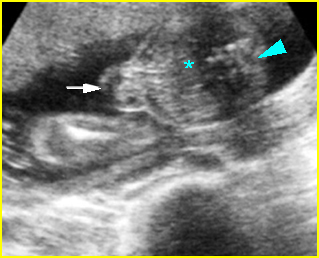
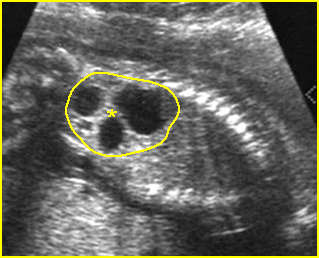
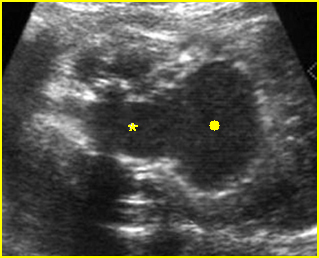
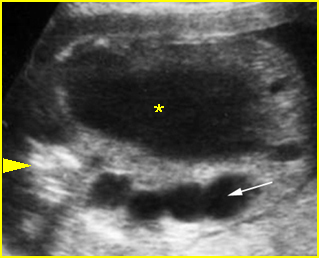
Fig 2: Normal thoracic spine Coronal scan of the thoracic spine: normal echodensity and alignment
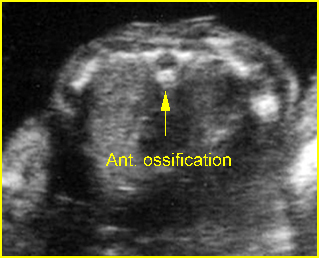
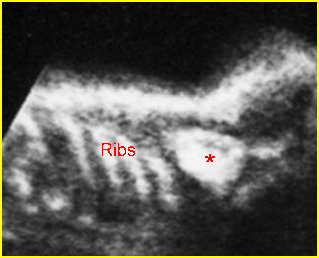
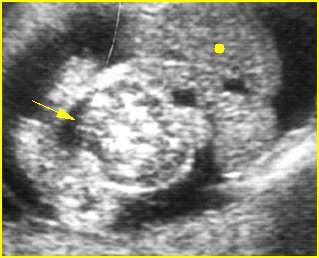
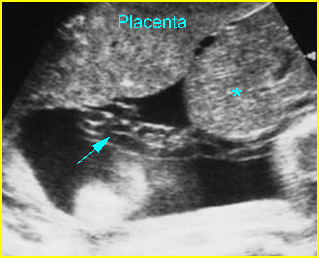
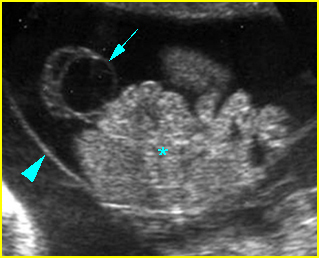
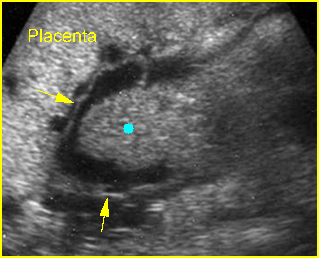
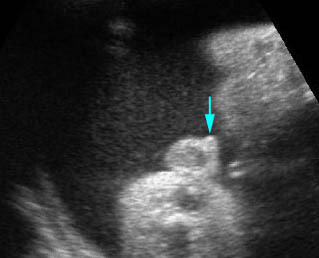
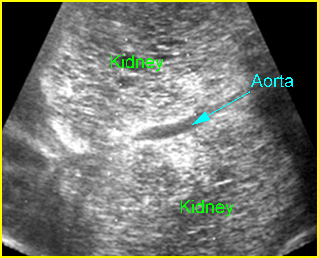
Fig 3: Normal spine : Cross-sectional scan of the abdomen posterior ossification centers of spine align horizontally (arrow = anterior ossification center)
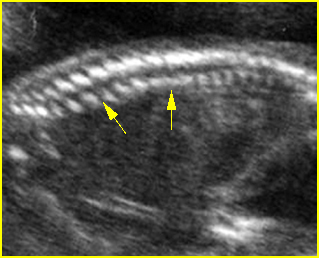
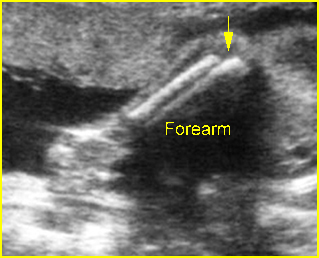
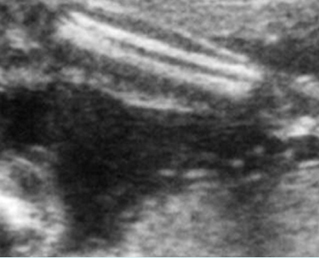
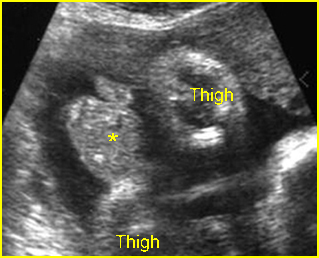
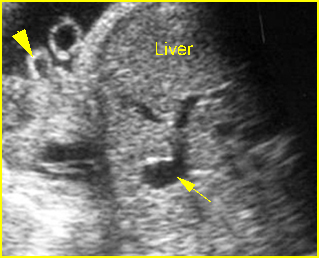
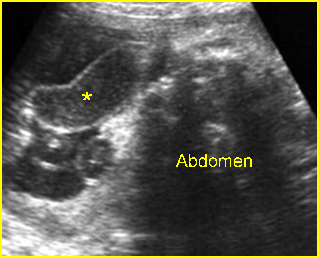
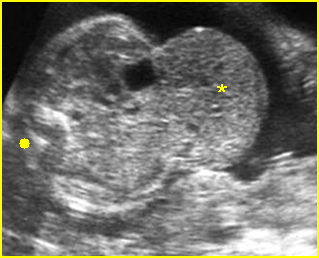
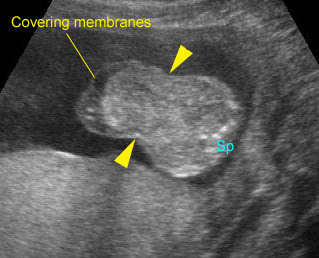
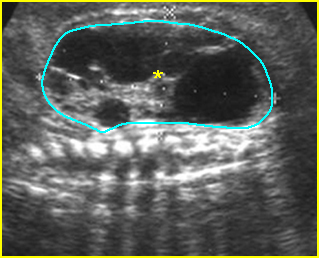
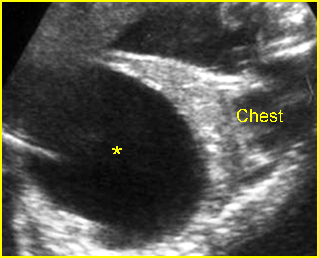
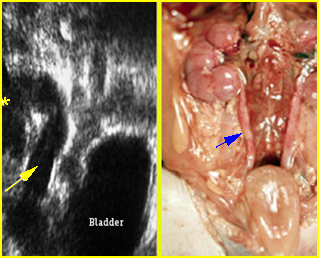
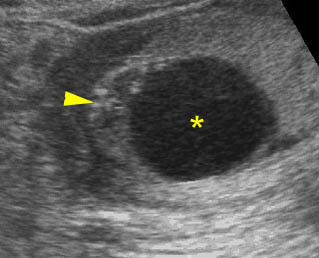
Fig 4: Normal spinal curvature Sagittal view of the spine: normal curvature (arrow) with complete overlying skin
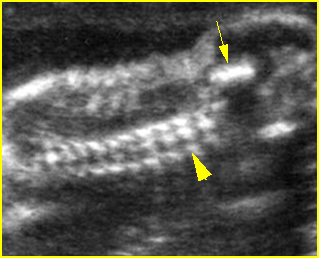
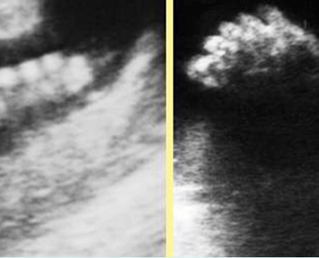
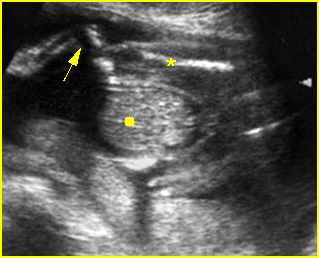
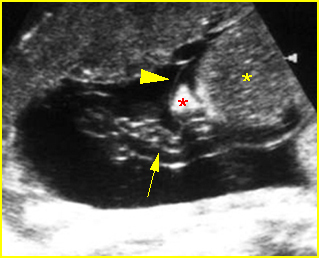
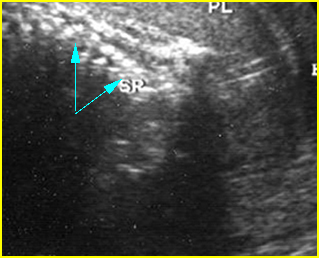
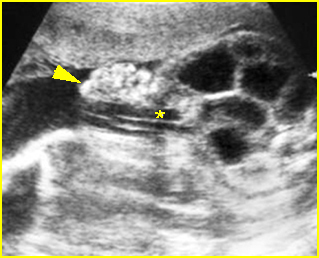
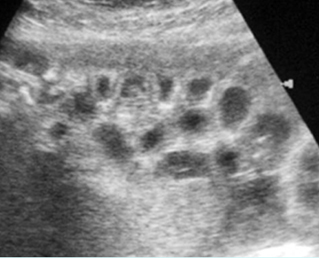
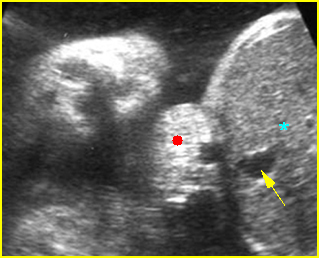
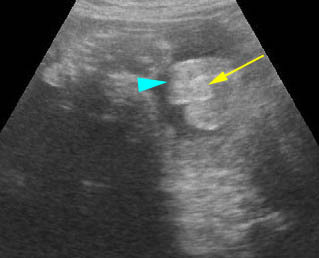
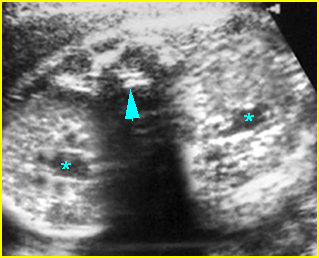
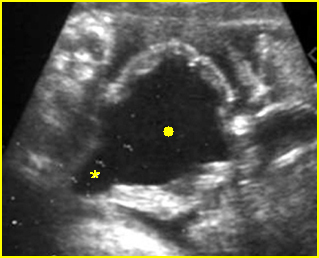
Fig 5: Normal lumbar spine Coronal scan of the lumbar spine (arrowhead) (arrow = iliac bone)
Video clips of normal examination
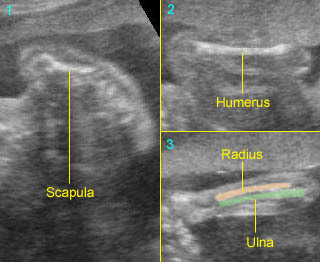
Normal spine (sagittal scan) : Rotating the transducer: from cross-sectional view (post. ceters in the horizontal plane) to sagittal view of the spine
Normal spine (coronal scan): Rotating the transducer: from cross-sectional view (post. ceters in the vertical plane) to coronal view of the spine
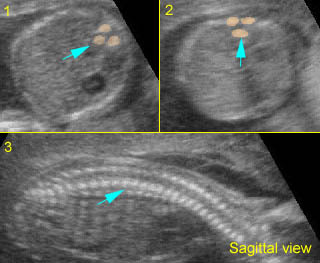
Normal spine (sagittal scan): Rotating the transducer: from cross-sectional view (post. centers in the horizontal plane) to sagittal view of the spine
(arrow = anterior ossification center)
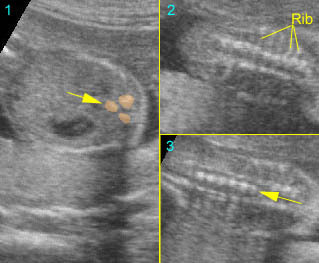
Normal spine (coronal view) : Rotating the transducer: from cross-sectional view (post. centers in the vertical plane) to coronal view of the spine
Potential pitfalls:
-
- Incomplete ossification: Scanning in early pregnancy reveals incomplete ossification of the lateral centers. Hence, on the transverse view, the posterior centers may appear to be parallel to one another rather than converging to the midline. This may be misinterpreted as spinal dysraphism.
- Pseudodyspharism: On the coronal plane of the cervical and lumbar spine, the two parallel lines of posterior centers normally diverge. This divergence should not be mistaken for a spinal dysraphism.
- Pseudodyspharism: <?please check the following sentence>On the transverse scan of the lumbosacral spine, if the ultrasound beam angled obliquely across the vertebral body of one vertebra but missed the posterior element or the posterior centers of another, this could simulate dyspharism. A normal appearance is restored when the ultrasound beam is reoriented perpendicular to the spinal axis.
- High-quality sonograms can also show the spinal cord within the spinal canal. The central canal is readily detectable within the cord. The more echogenic cauda equina (nerve roots) can be seen distal to the conus medullaris (distal spinal cord). With growth of the spine, the position of the conus medullaris ascends with gestational age.
- The spinal cord neural tissue, like that of most brain tissue, is echopenic. The conus medullaris and the craniocervical junction can be seen, albeit inconsistently, in nearly all fetuses by 18-20 weeks. The tissues surrounding the cord (leptomeninges) are brightly echogenic, as are those that surround the brain, and the dura is usually also seen discretely as a linear bright reflector. In fetuses with myelomeningoceles, determination of the most cephalic spinal level lesion is an important factor in prognosis. This level can be determined by counting up from the last ossified vertebral segment.