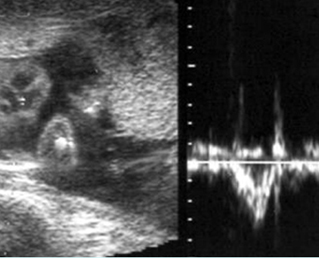
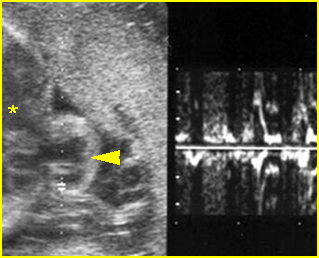
Fetal Cardiac Arrhythmias
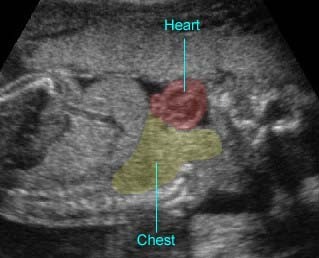
Fetal arrhythmia is abnormal fetal heartbeats, including an irregular rhythm, tachycardia and bradycardia. Utilizing M-mode echocardiography, both fetal atrial and ventricular contractions can be evaluated for regularity and their relationship to each other in time. The most common important fetal arrhythmias are: (1) supraventricular tachycardias; and (2) severe bradyarrhythmias, associated with complete heart block. These may be associated with the development of hydrops fetalis.
Incidence: 1-2% of pregnancies. In clinical practice, these are commonly seen in fetuses whose mothers have a high intake of caffeine. Cessation of that intake often eliminates this arrhythmia.
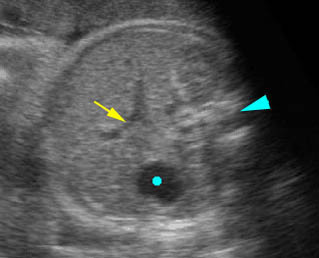
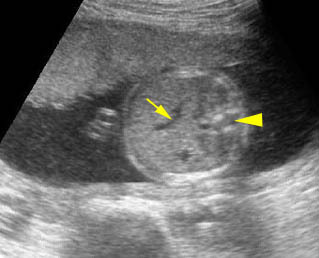
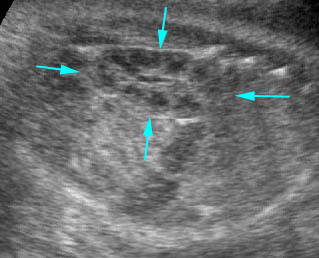
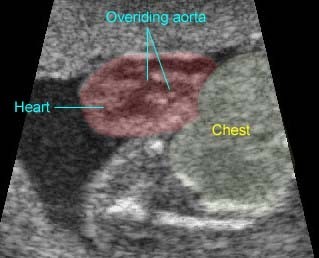
Fig 1, Fig 2, Fig 3, Fig 4, Fig 5
Irregular rhythms:
- The most common type is premature atrial contractions.
- They may be associated with maternal drug use, including hydralazine, nifedipine, as well as caffeine.
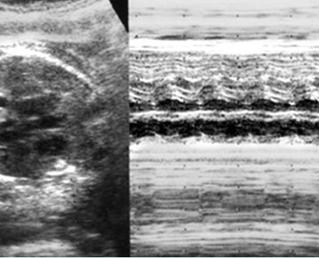
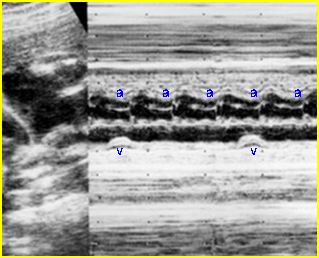
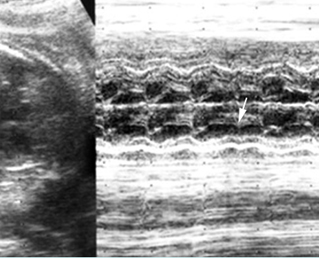
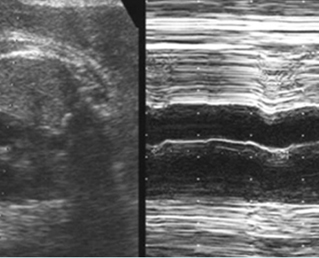
- Diagnosis: With M-mode or Doppler ultrasound, the cursor is placed through the atrial wall and ventricular wall, and the fetal heart rate and regularity can be evaluated. Premature atrial contractions may be either conducted to the ventricles or blocked, both resulting in an increased ventricular rate.
- Cardiac and other associated anomalies are rare.
- Slightly increased risk of developing supraventricular tachycardia.
- Prognosis: Almost always benign and requires no treatment. Those without persistent irregularities can be followed up with routine prenatal care.
Tachycardia
- Tachycardia refers to a fetal heart rate faster than 160 bpm.
- With sinus tachycardia, the impulse originates from the sinus node, usually ranges between 180 and 200 bpm and is commonly related to changes in autonomic tone, including those caused by maternal fever or drugs.
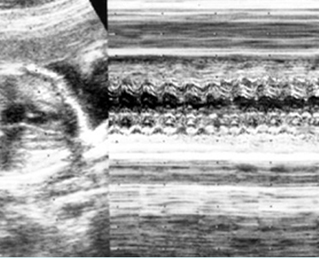
- Supraventricular tachycardia (SVT) , a re-entry tachycardia involving an accessory pathway between the atrium and the ventricle, is the most common type of sustained tachycardia with a rate of 220-280 bpm and a 1:1 association between the atrial and ventricular contractions.
- Atrial flutter, a single re-entry circuit within the atria, refers to a rate that ranges from 300 to 600 bpm, with 2:1 atrioventricular block.
- Ventricular tachycardia is a rare arrhythmia associated with atrioventricular dissociation, with the ventricular rate (150-250 bpm) faster than the atrial rate.
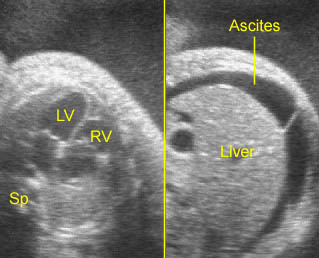
- Hydrops fetalis can occur in approximately 50% of fetuses with sustained tachycardia.
- The fetuses with sustained tachycardia need in utero treatment. The hydropic fetuses with unsuccessful in utero therapy may need early delivery.
- Prognosis: The survival rate of the fetuses with SVT depends on the development of hydrops. 50-80% of those with hydrops can be in utero controlled with currently available drugs such as sotolol, flecainide, and amiodorane; however, these fetuses may have already developed ischemic cerebral complications that may not be completely resolved. In about 50% of fetuses with tachycardia, the condition persists or recurs postnatally and needs long-term treatment.
Bradycardia
- Bradycardia is defined as a ventricular rate <110 bpm, often associated with extracardiac causes, such as fetal distress, maternal drugs, or cord compression.
- Sinus bradycardia, typically 70-110 bpm, in which the impulse originates at the sinus node with 1:1 atrioventricular association, is usually well tolerated.
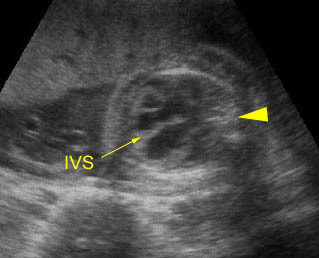
- Second degree heart block refers to incomplete penetration of the sinus beat through the AV node and bundle of His.
- Complete heart block (third degree), a complete dissociation between atrial and ventricular contraction, probably accounts for 80% of cases. About 50% are associated with structural cardiac defects, especially heterotaxy or corrected transposition, and most of the remainder are associated with maternal autoantibodies, such as anti-SSA and SSB.
- Hydrops fetalis can be seen in about one-third of cases in which the ventricular rate is usually <55 bpm.
- In utero treatment: A beta agonist, such as salbutamol, can increase the fetal heart rate significantly and improve ventricular function or even reverse hydrops fetalis. Furthermore, dexamethasone can also successfully be used to reverse the heart block to a lesser degree.
- The prognosis depends on the presence of structural cardiac defects or hydrops. The prognosis is generally poor in those with long QT syndrome manifested as intermittent fetal bradycardia and tachycardia with AV dissociation, though some cases have a favorable outcome.
Conditions commonly associated with conduction defects that produce arrhythmias
- Tetralogy of Fallot
- Double-outlet right ventricle
- Transposition of the great vessels
- Pulmonic stenosis
- Total anomalous pulmonary venous return.