Duodenal Atresia
Duodenal Atresia
Duodenal atresia is the most common type of congenital small bowel obstruction resulting from failure to recanalize the duodenal lumen during the 11th week of gestation.
Fig 1
Incidence: 1 in 5000-10,000 births. One-third have Down’s syndrome.
Sonographic findings:
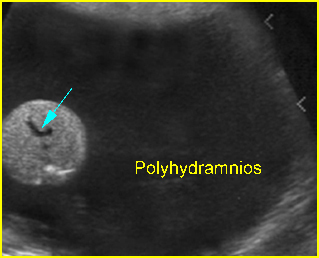
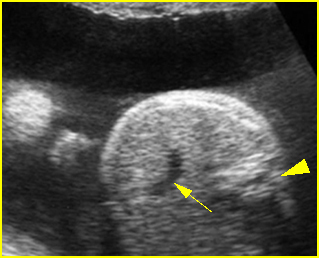
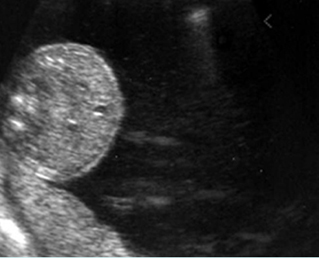
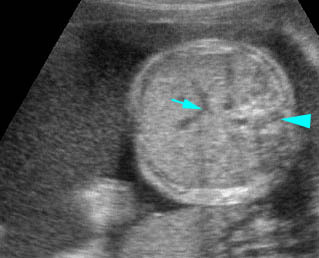
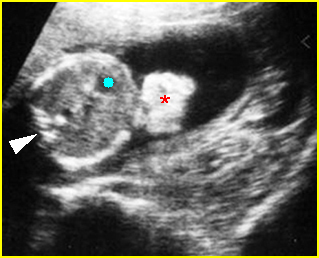
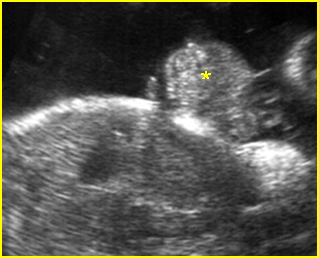
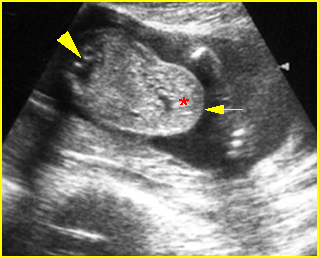
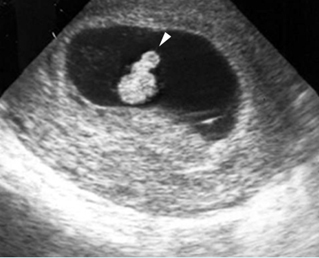
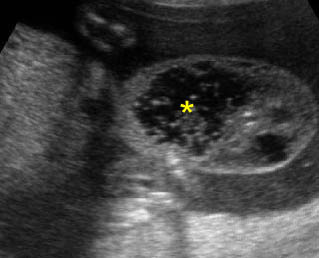
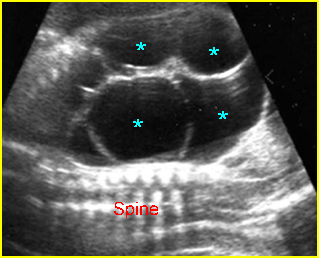
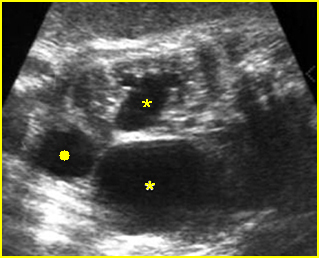
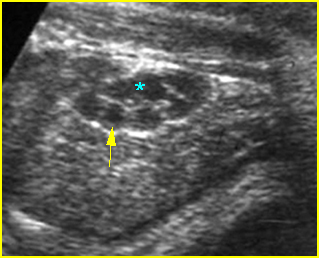
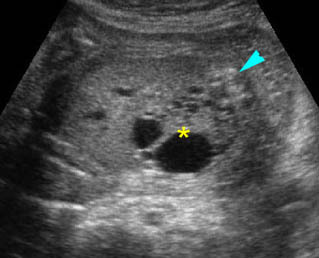
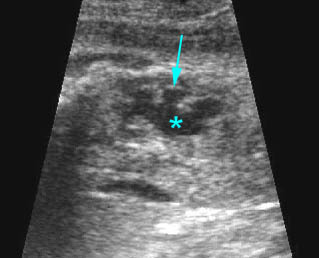
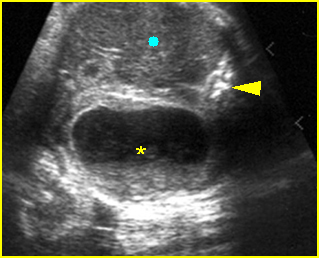
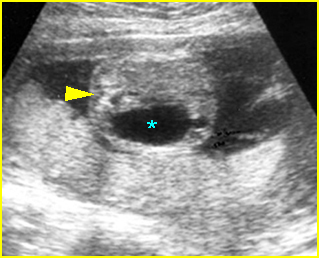
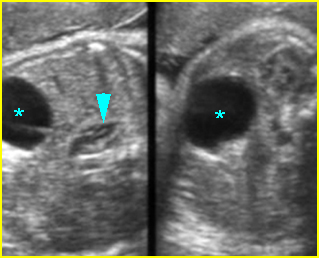
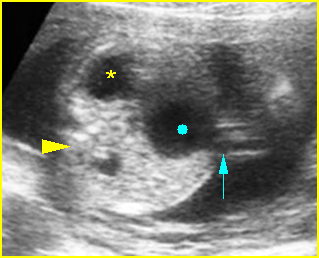
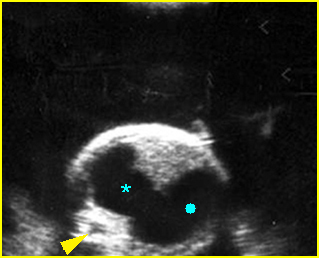
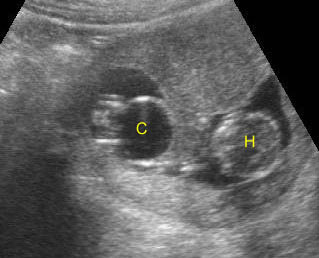
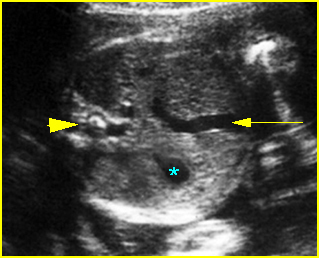
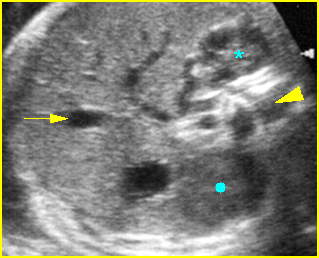
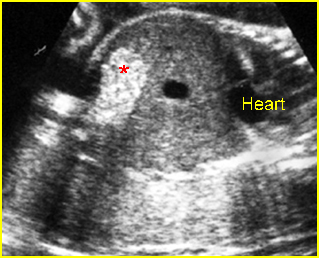
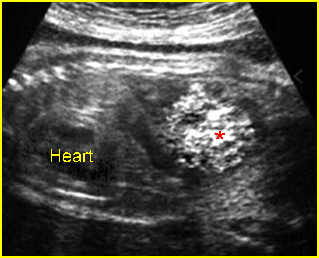
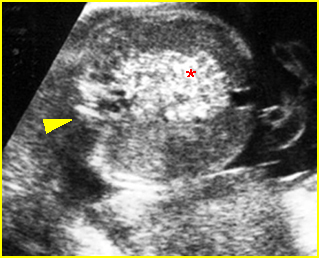
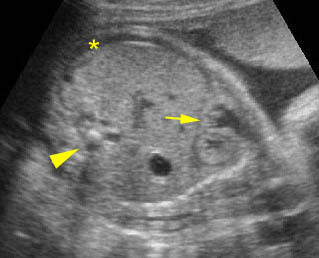
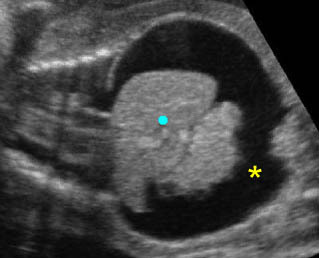
- Double bubble sign and a narrow channel connecting the stomach and duodenum (the second bubble will be in the location of the duodenal bulb, which is typically in the center of the abdomen, just to the right of the midline). Fig 2, Fig 3, Fig 4
- The continuity between the two fluid-filled structures must be demonstrated, and if the connection cannot be shown, a number of other possibilities should be considered, such as choledocal cyst (most common) and the less common renal cyst, hepatic cyst, omental cyst, bowel duplication or ovarian cyst.
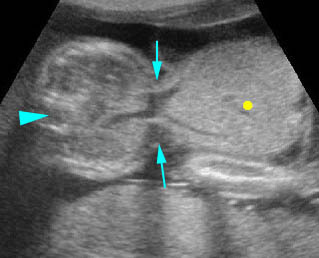
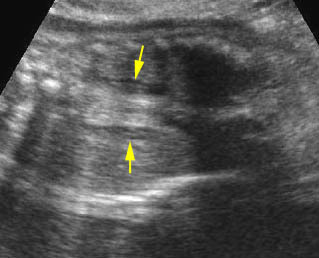
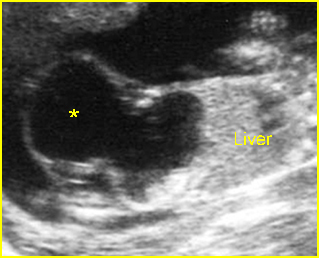
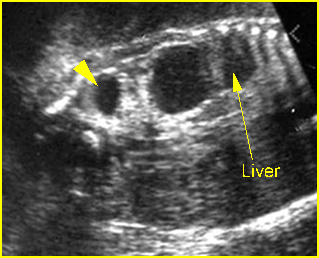
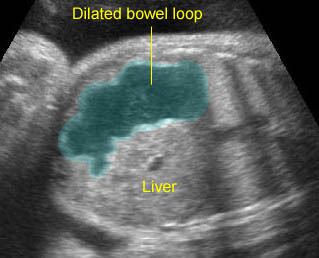
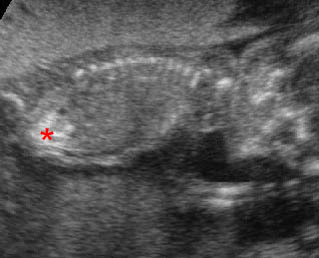
- A more prominent appearance with a C loop of dilated bowel may occur when duodenal atresia is associated with esophageal atresia. Fig4, Fig5
- Pitfalls:
- A prominent normal stomach with a visible incisura angularis may be confused with a double bubble sign.
- Bisection of the normal stomach in an oblique scan plane, giving the spurious appearance of a double bubble. This misinterpretation can be corrected by scanning the abdomen in a true transverse plane, so as to demonstrate the typical tapering configuration of the gastric antrum.
- Any right upper quadrant mass such as a choledocal cyst, bile-filled gallbladder, or hepatic cyst may be misinterpreted as a distended duodenum.
- Polyhydramnios occurs in nearly all cases diagnosed in the third trimester.
- Perforation secondary to obstruction can occur, and ascites or meconium peritonitis may be found.
- Usually first diagnosable in the second trimester, although it can occasionally be diagnosed before 20 weeks or even in the first trimester.
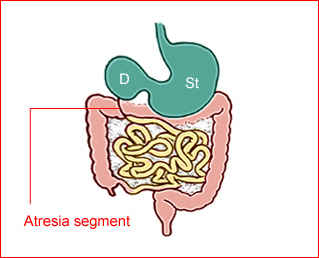
Fig 1: Schematic drawing of duodenal atresia, The fluid-filled stomach (St) and duodenum (D) cause double bubble sign on ultrasound examination
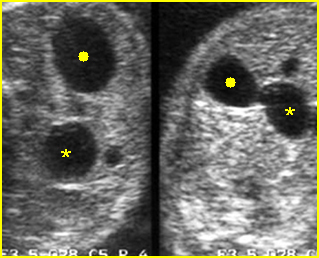
Fig 2: Duodenal atresia Cross-sectional scan of the abdomen: Double bubble sign: two cystic masses (* = proximal duodenum, solid circle = stomach) with connection together in the upper abdomen

Fig 3: Duodenal atresia Cross-sectional scan of the abdomen: Double bubble sign: two cystic masses (* = stomach, solid circle = proximal duodenum ) in the upper abdomen
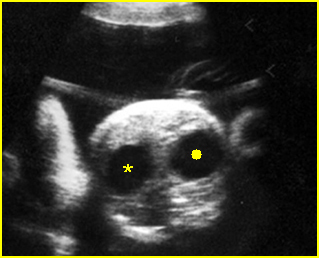
Fig 4: Duodenal atresia Cross-sectional scan of the abdomen: Double bubble sign: two cystic masses (* = proximal duodenum, solid circle = stomach) in the upper abdomen
Video clips of duodenal atresia
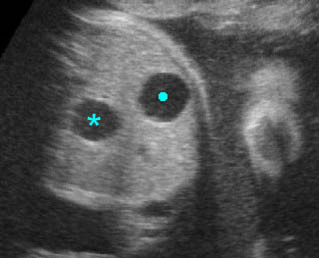
Double bubble sign: Cross-sectional scan of the abdomen: two connecting cystic masses (*) in the upper abdomen with polyhydramnios
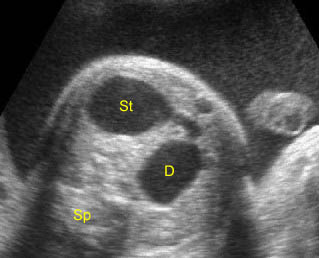
Duodenal atresia: Double bubble sign with continuation representing stomach (St) and duodenum (D) (Sp = spine)

Duodenal atresia: C-loop of duodenum and stomach secondary to duodenal and esophageal atresia
Associations: Gastrointestinal, genitourinary and cardiovascular anomalies occur in more than 50% of cases and trisomy 21 is found in 30% of cases (but only 5% of fetuses with trisomy 21 show duodenal atresia). Furthermore, duodenal obstruction also increases the risk of prenatal asphyxia and death, probably caused by bradycardia/asystole following vagal overactivity due to distension of the upper gastrointestinal tract.
Management: A careful search for associated anomalies and karyotyping are indicated. The delivery should occur where appropriate personnel are available for surgical correction.
Prognosis: A high mortality rate of about 20-40% due to associated abnormalities or preterm delivery. Uncomplicated cases have a good prognosis.
Recurrence risk: Sporadic (with rare familial reports).