Hydronephrosis
Hydronephrosis
Hydronephrosis is the dilatation of the fetal renal collecting system. It is the most common fetal abnormality detected by antenatal ultrasound. It can result from ureteropelvic junction (UPJ, 41%), ureterovesical junction (UVJ, 23%), duplication of the collecting system (13%), and bladder outlet (10%). Renal dysplasia can occur in some fetuses with severe or early-onset obstruction.
UPJ obstruction is a functional obstruction and is bilateral in 30% of cases. It affects males more than twice as often as females. Most cases do not become dysplastic.
Vesicoureteral reflux is much more common among males and is often bilateral. It often resolves within the first years of life. Reflux that does not resolve spontaneously or is very severe at birth can be corrected surgically.
Incidence: 1-5 per 1000 births (ureteropelvic junction obstruction: male/female, 4:1, ureterovesicular junction: male>female).
Sonographic findings:
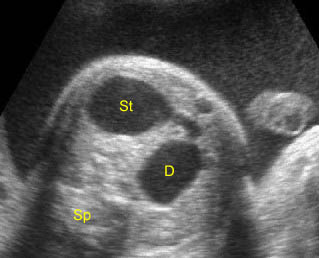
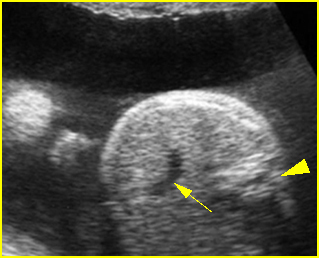
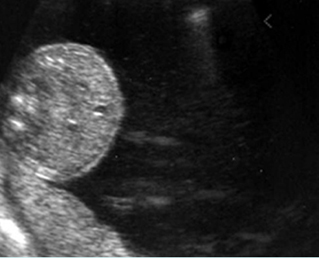
- A diagnosis of hydronephrosis should be made when there is calicectasis or the antero-posterior diameter of the renal pelvis, measured on a transverse view through the kidney, is at least 8 mm at 16-20 weeks or at least 10 mm after 20 weeks of gestation. Fig 1, Fig 2, Fig 3, Fig 4, Fig 5 Fig 6, Fig 7
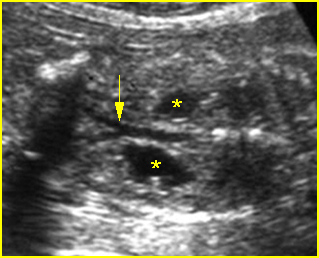
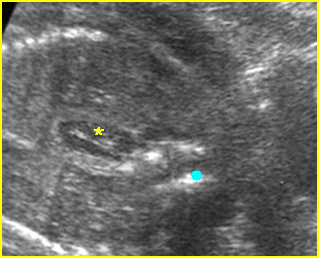
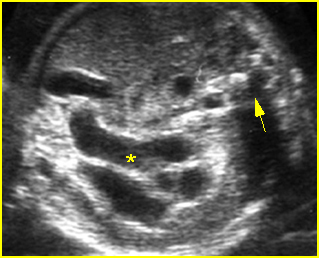
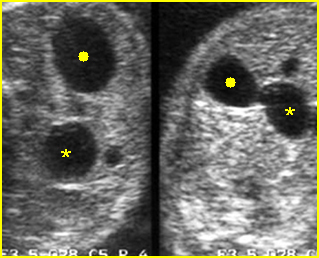
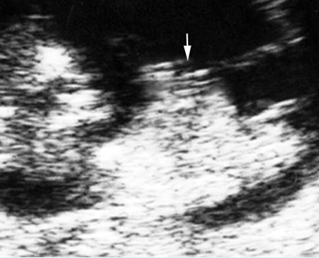
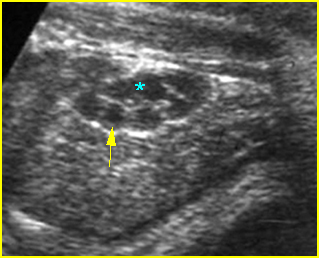
Fig 1: Hydronephrosis Coronal scan of the posterior abdomen: mild dilated renal pelvis (*) (arrow = bifurcation of aorta)
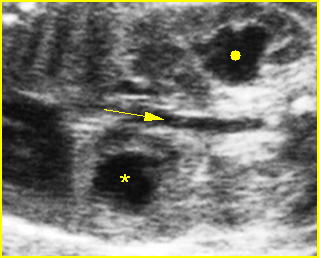
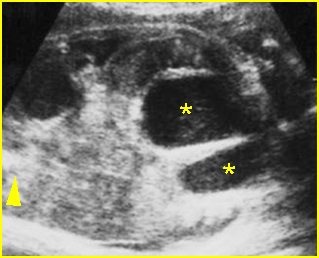
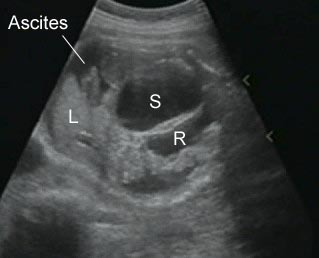
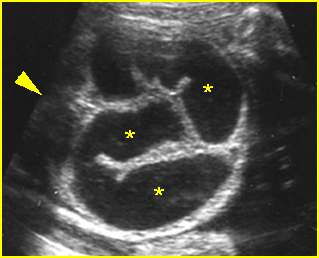
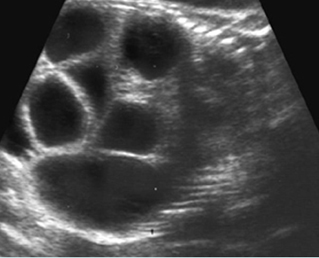
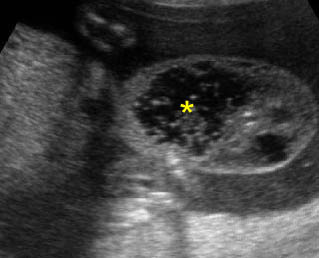
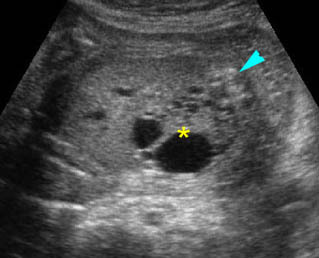
Fig 2: Hydronephrosis Coronal scan of the abdomen: bilateral markedly dilated renal pelvis (*) (arrow = aorta)
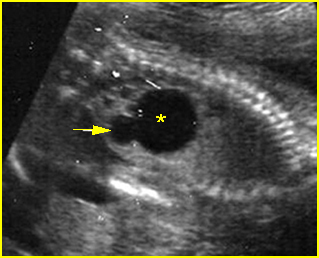
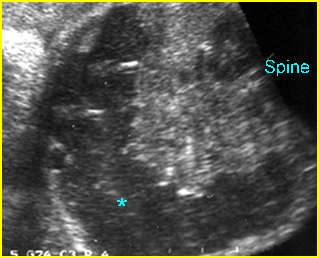
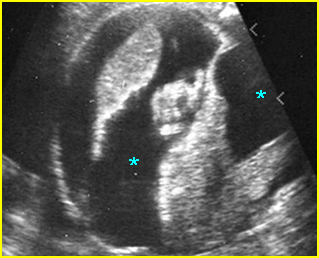
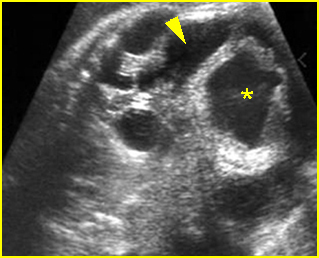
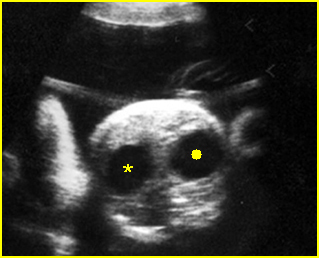
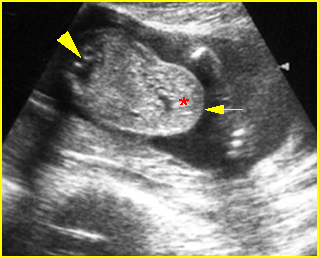
Fig 3: Hydronephrosis Sagittal scan of the abdomen: markedly dilated renal pelvis (*) and calyx (arrow)
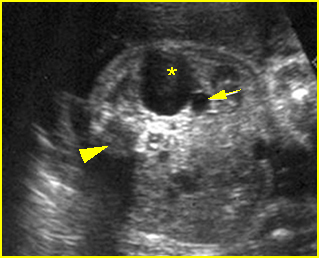
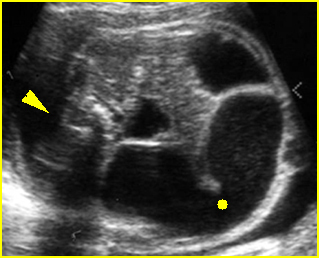
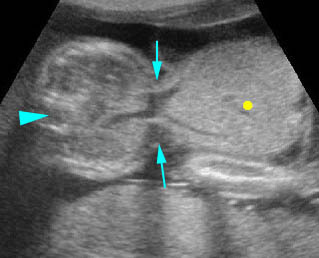
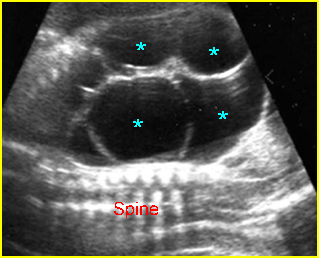
Fig 4: Hydronephrosis Cross-sectional scan of the abdomen: markedly dilated renal pelvis (*) and calyx (arrow) (arrowhead = spine)
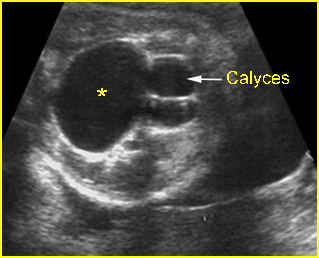
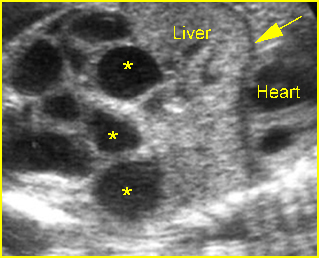
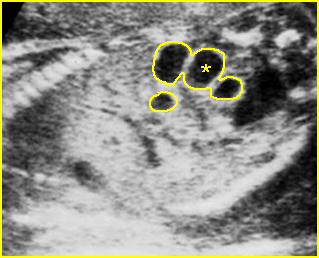
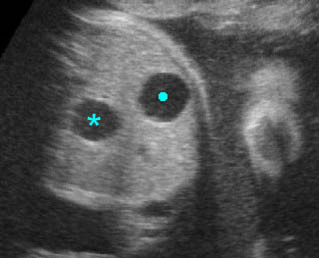
Fig 5: Hydronephrosis Cross-sectional scan of the abdomen: markedly dilated renal pelvis (*) with calyces (arrow)
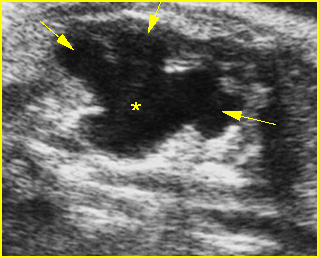
Fig 6: Hydronephrosis Parasagittal scan of the abdomen: markedly enlarged renal pelvis (*) and calyces (arrow)
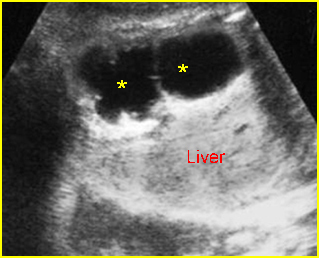
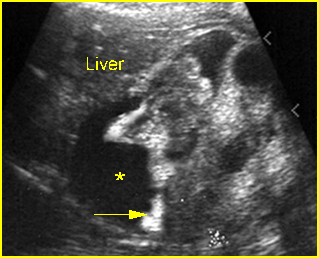
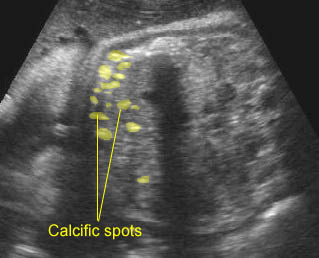
Fig 7: Severe hydronephrosis Sagittal scan of the abdomen: markedly enlarged renal pelvis (*) with echogenic thin parenchyma
Video clips of large hydronephrosis
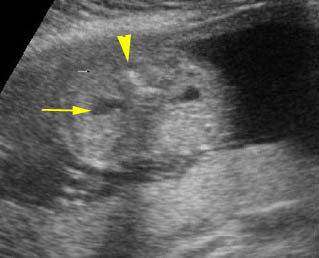
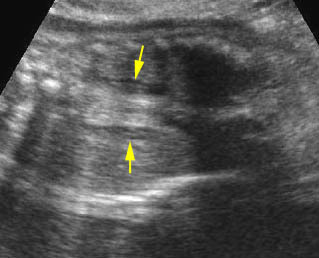
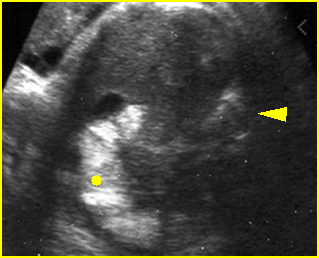
Mild pyelectasis: Cross-sectional scan of the abdomen: bilateral mild dilatation of renal pelvis (arrow) (arrowhead = spine)
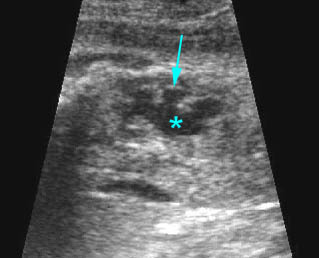
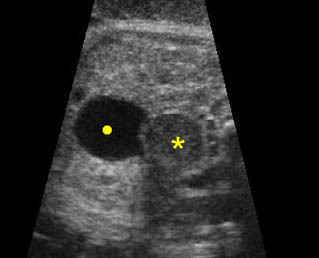
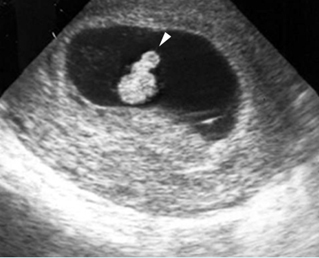
Hydronephrosis: Sagittal scan of the fetal kidney: dilatated renal pelvis (*), and calyces (arrow)
- The Society for Fetal Urology recommends a scoring system to grade dilation of the upper urinary tract as follows:
- Grade 0: no dilation Fig 8
- Grade I: renal pelvic dilation with or without infundibula visible Fig 9
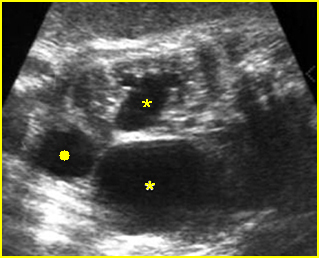
- Grade II: renal pelvic dilation with calices visible Fig 10, Fig 11
- Grade III: renal pelvis and calyces dilated Fig 12
- Grade IV: features of grade III with parenchymal thinning Fig 13
- Ultrasound grading of hydronephrosis correlates with the severity of cortical damage or the decrease in renal function on postnatal renal scan.
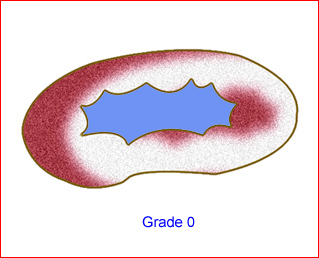
Fig 8: Schematic drawing of grade 0: no dilation
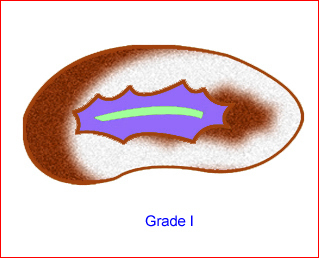
Fig 9: Schematic drawing of grade I: renal pelvic dilation without calices visible
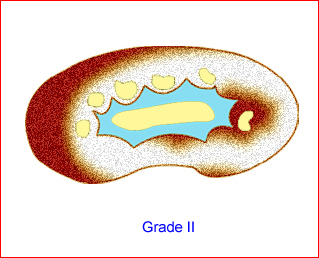
Fig 10: Schematic drawing of grade II: renal pelvic dilation with calices visible
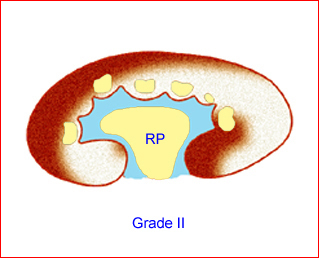
Fig 11: Schematic drawing of grade II: renal pelvic dilation with calices visible
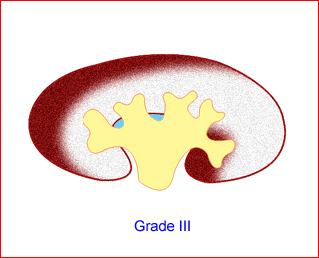
Fig 12: Schematic drawing of grade III: renal pelvis and calices dilated
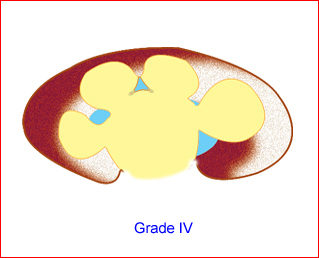
Fig 13: Schematic drawing of grade IV: renal pelvis and calices dilated as well as parenchymal thining
- Proposed criteria for the diagnosis of fetal hydronephrosis in different studies:
- Arger (1985): AP diameter >10 mm or ratio of AP diameter of pelvis/kidney >0.5
- Corteville (1991: AP diameter >4 mm at <33 weeks or >7 mm at >33 weeks, or ratio of AP diameter of pelvis/kidney >0.28
- Mandell (1991): AP diameter >5 mm at 20 weeks, or >8 mm at 20-30 weeks, or >10 mm at >30 weeks
- Adra (1995): AP diameter >8 mm from 28 weeks to term
- Ouzounian (1996): AP diameter >5 mm from 16 weeks to term
- Dudley (1997): AP diameter >5 mm from 16 weeks to term.
- UPJ obstruction: The ureters are not dilated and the amniotic fluid volume is usually normal.
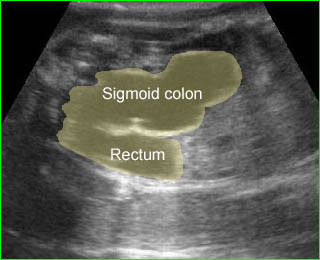
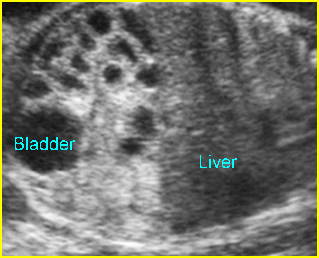
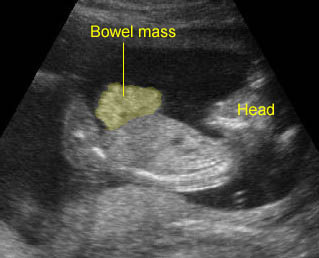
- Vesicoureteral reflux: the hydroureter will be visualized. In severe cases, the ureter may be markedly dilated and tortuous. Care must be taken not to mistake such a ureter for a dilated bowel loop. This error can be avoided by following the dilated ureter proximally to the renal pelvis and distally to the bladder.
- Dysplastic kidney, which possibly occurs with UPJ obstruction, can be diagnosed when the parenchyma becomes abnormally echogenic or cystic.
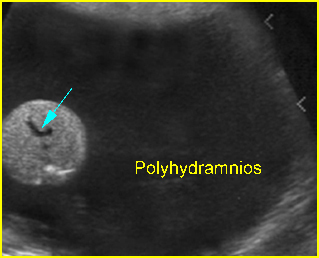
- The contralateral kidney and related obstructions, such as ureteral dilatation, bladder distension, urethral dilatation and amniotic fluid, must be carefully evaluated.
Associations: A renal pelvis dilated to >4 mm increases the risk of trisomy 21, 18, 13.
Management: Serial sonographic monitoring is necessary to assess the degree of severity. In cases of severe obstruction, early delivery may be beneficial and the delivery should occur in a tertiary center since neonatal evaluation and treatment are necessary.
Prognosis: Good for mild forms of isolated cases, but poor in cases of dysplastic kidney especially secondary to prolonged bladder outlet obstruction. Mild fetal hydronephrosis (>4 mm) at mid-trimester appears to be associated with an excellent prognosis, probably representing physiological renal pelvic dilatation. Most cases resolve before delivery. Moderate/severe (>7 mm) fetal hydronephrosis is associated with a poorer outcome.
Recurrence risk: Sporadic in the absence of a recognizable syndrome.