Transposition of the Great Arteries (TGA)
Transposition of the Great Arteries (TGA)
The aorta arises from the right ventricle and lies anterior to and left of the pulmonary artery, which is connected to the left ventricle and lies posteriorly and medially. Complete TGA is uneventful in utero. Survival after birth depends on the amount and size of the mixing of two otherwise independent circulation systems. In the case of an intact ventricular septum, the newborn presents shortly after birth with cyanosis and deteriorates rapidly.
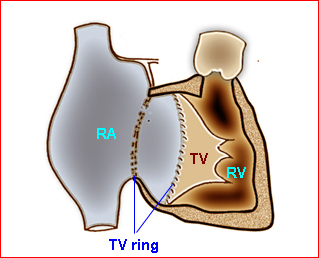
Fig 1
Incidence: 2 per 10,000 live births, accounting for 5% of CHD.
Sonographic diagnosis:
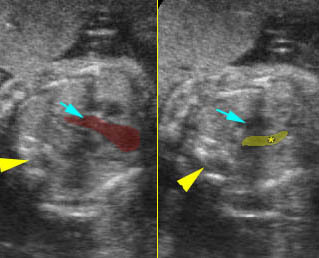
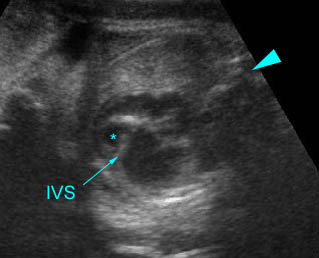
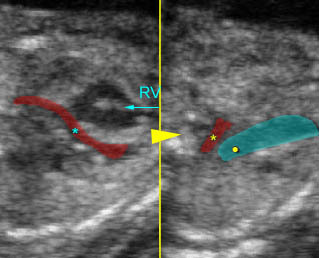
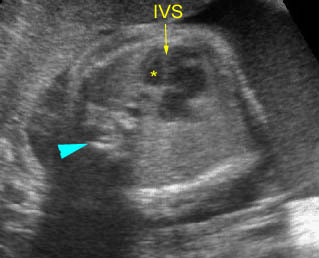
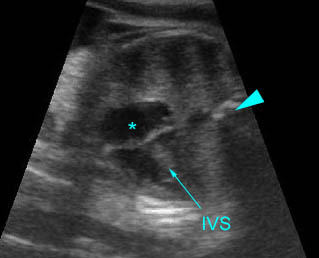
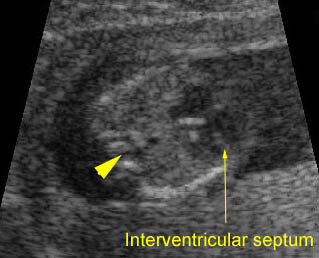
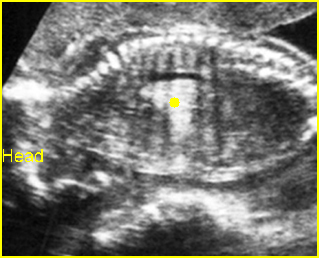
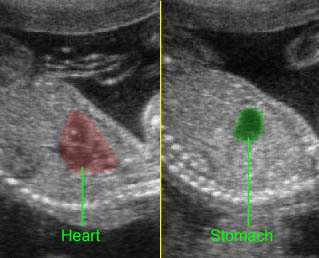
- The aorta and pulmonary artery are parallel instead of crossing at the level where they arise from the ventricles.
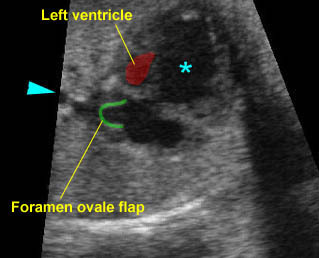
- The vessel connected to the left ventricle has a posterior course and bifurcates into two pulmonary arteries.
- The vessel connected to the right ventricle has a long upward course and gives rise to the brachiocephalic vessels.
- Other cardiac anomalies, especially VSD or pulmonary stenosis.
- Atrioventricular block is common.
- Doppler study may show abnormal pulmonary venous blood flow velocity waveforms, and lower pulsatility indices in the middle cerebral artery.
- Pitfalls:
- It can often be confused with double-outlet right ventricle or tetralogy of Fallot.
- TGA can be missed if the main pulmonary artery is not distinguished from the ascending aorta.
- In cases of mild or isolated forms or corrected TGA, the screening ultrasound may show only an isolated VSD or a small VSD, with a tiny pulmonary trunk.
- Usually diagnosed after 16-18 weeks, but possible after 13-15 weeks with transvaginal ultrasound in some cases.
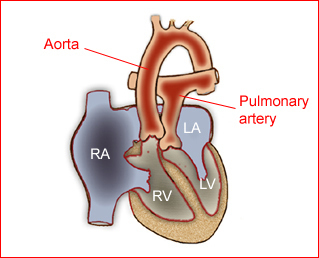
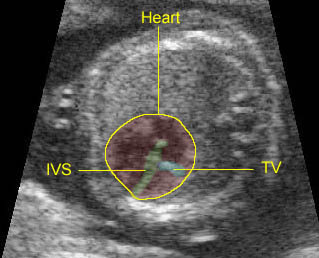
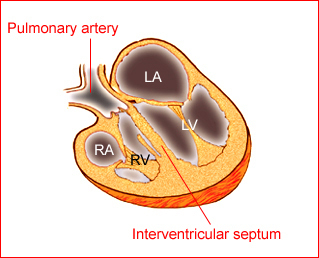
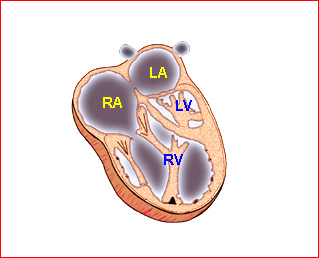
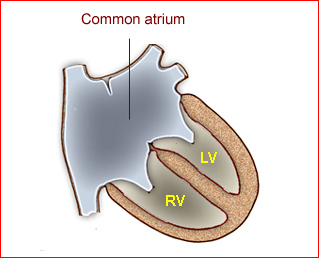
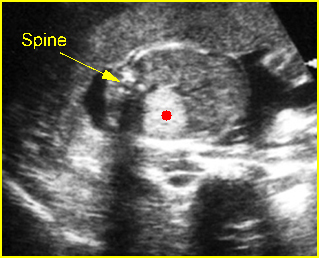
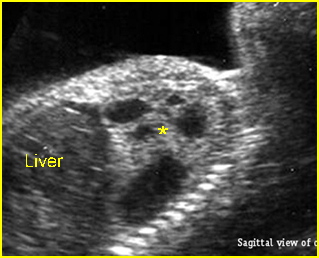
Fig 1: Schematic drawing of transposition of the great arteries (LA = left atrium, LV = left ventricle, RA = right atrium, RV = right ventricle)
Video clips of Transposition of the Great Arteries (TGA)
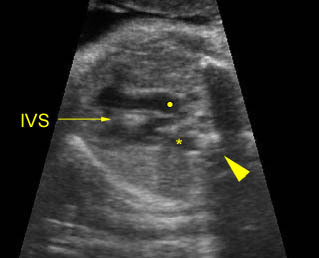
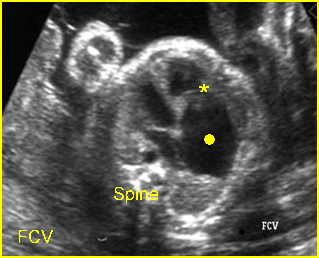
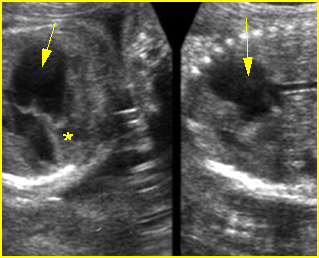
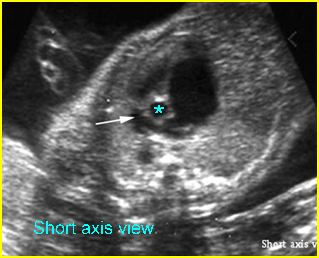
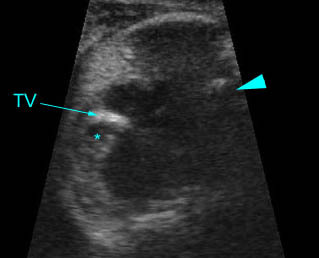
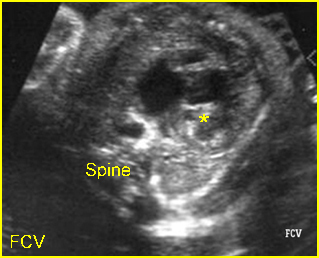
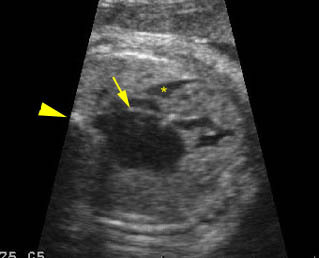
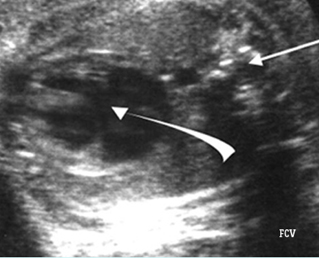
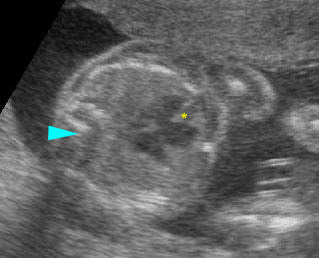
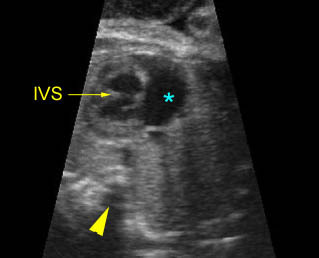
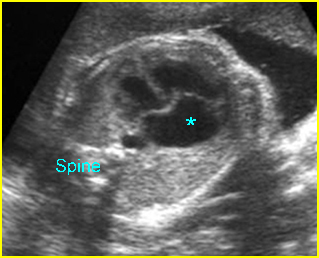
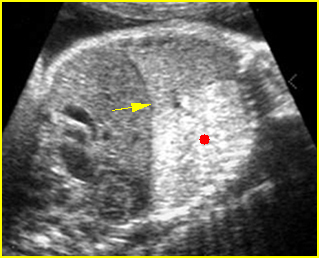
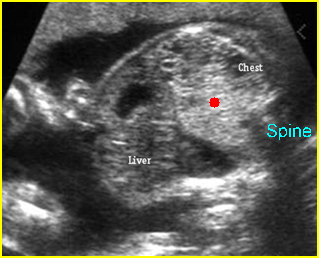
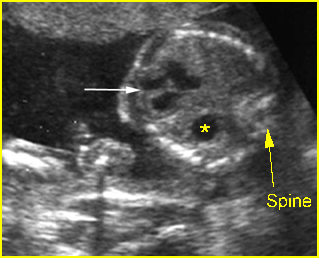
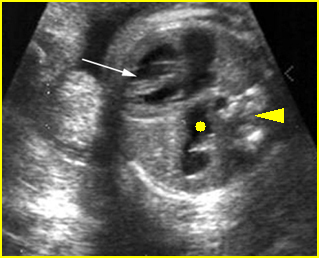
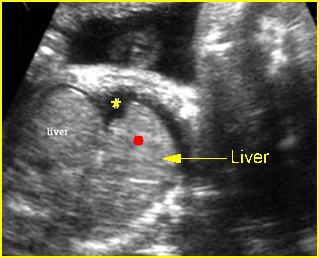
Transposition of the great vessels: Long-axis view: aortic root (solid circle) and pulmonary trunk (*) parallel arising from right and left ventricle respectively (arrowhead = spine)
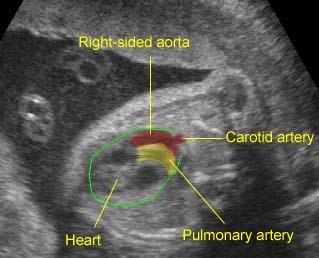
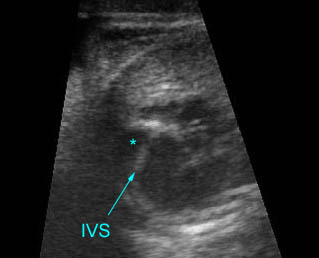
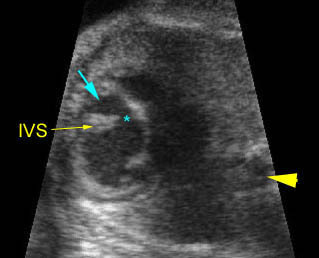
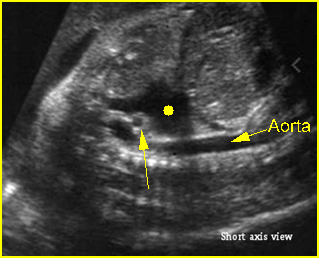
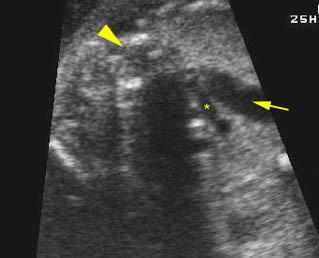
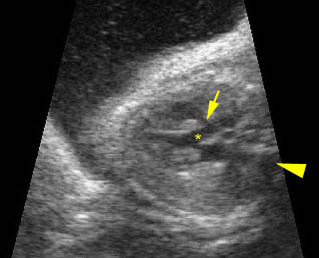
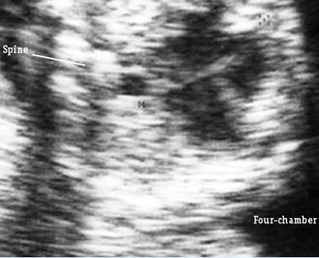
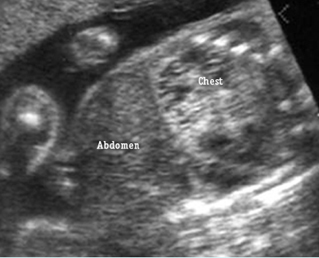
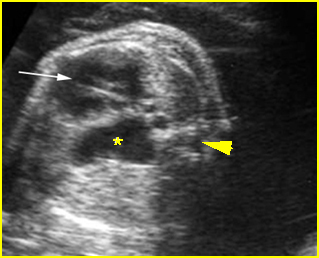
Transposition of the great arteries: Long axis view: the right-sided aorta running parallel to the left-sided pulmonary artery. Note: the carotid artery arising from the aortic arch
Associations: Other cardiac defects are seen in 50% of cases.
Management: Careful prenatal and postnatal search for associated anomalies and karyotype (including FISH for chromosome 22q11 deletion) are indicated. Delivery should be performed where immediate pediatric cardiac consultation is available.
Prognosis: Complete TGA is well tolerated in utero. Postnatal surgery improves the prognosis, however, the mortality rate is still high overall, especially when associated with other abnormalities. Prenatal diagnosis may improve the preoperative condition of the neonates.